
��������(CeO2)��һ����Ҫ��ϡ�������ƽ�������ʾ�����������в��������ķϲ�����ĩ(��SiO2��Fe2O3��CeO2�Լ���������������ϡ�������)��ij�������Դ˷�ĩΪԭ�ϻ����棬���ʵ���������£�
(1)ϴ������A��Ŀ����Ϊ��ȥ��________(�����ӷ���)������������Ƿ�ϴ���ķ�����___________________________________________________��
(2)�ڢڲ���Ӧ�����ӷ���ʽ��__________________________________������B����Ҫ�ɷ���________��
(3)��ȡ�Ƿ���ϡ��Ԫ�صij��÷�������֪������TBP��Ϊ��ȡ���ܽ������Ӵ�ˮ��Һ����ȡ������TBP________(��ܡ����ܡ�)��ˮ���ܡ�ʵ���ҽ�����ȡ����ʱ�õ�����Ҫ����������________���ձ�������������Ͳ�ȡ�
(4)ȡ���������еõ���Ce(OH)4��Ʒ0.536 g���������ܽ����0.100 0 mol��L��1 FeSO4����Һ�ζ��յ�(�汻��ԭΪCe3��)������25.00 mL����Һ���ò�Ʒ��Ce(OH)4����������Ϊ________��
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���乤���������£�
(1)����۵����ӷ���ʽ�� ��
(2)�������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣���������������� ��
(3)��ˮ������������һ����������������ڲ�����пɽ��������������ͨ�� (���Լ�����)��Һ���Գ�ȥ������
(4)����ݵ���������У��¶�Ӧ������80��90 �档�¶ȹ�����Ͷ������������������ԭ�� ��
(5)��������������ữ�����Cl2�������ʣ������� ��
(6)��ȡ�嵥�ʣ�����������ˮ����������������ˮ��������˵��ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ���ۺ����ÿ����Ʊ�����þ������������ͼ��ʾ��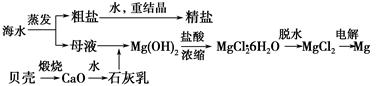
(1)Mg(OH)2�����л��е�Ca(OH)2Ӧ������ȥ��д��ʵ�鲽�衣__________________________
(2)ʵ���ҽ������Ƴɾ��εĹ����У��ܽ⡢���ˡ�������������IJ�����Ҫ�õ����������ֱ�˵���������������ʹ�ò�������Ŀ�ģ�
���ܽ⣺________________��
�ڹ��ˣ�__________________________��
��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Һ�žú���������������AgN3��������ը��ֱ���ŷŻ���Ⱦ���������������Դ���˷ѡ�ij�о�С������˴�������Ӧ��ķ�Һ�У���������������Һ�����費��������������������������ʵ�����̣�
����֪��[Ag��NH3��2]������Һ�д���ƽ�⣺[Ag��NH3��2]��??Ag����2NH3��
��1��д�������ڢٲ���Һ��ϡHNO3��Ӧ�����ӷ���ʽ ��
��2�������ڢڲ����������Ҫ������Ŀ���� ��
�������̿��ܲ����Ĵ�����Ⱦ���� ��
��3���ҷ��������յõ����۵�����ƫ���ų�δϴ�Ӹɾ������أ����ܵ�ԭ���� ��
��4��ʵ��������������Һ�IJ��������� ��
��5����֪�ҷ����ڢ۲���Ӧ��H2S��������������յõ�����21.6 g��������������ʧ�������ϸò���Ҫ�������� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�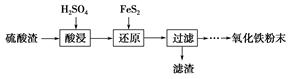
(1)�������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ���________��
(2)����ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(3)Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��ȷ��ȡһ���������������Һ����ƿ�У�����ϡ���ᡢ�Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ�Ļ�ѧ����ʽ���£�
2Fe3����Sn2����6Cl��=2Fe2����SnCl62��
Sn2����4Cl����2HgCl2=SnCl62����Hg2Cl2��
6Fe2����Cr2O72����14H��=6Fe3����2Cr3����7H2O
����SnCl2����������ⶨ��Fe3����________(�ƫ�ߡ�����ƫ�͡����䡱����ͬ)��
��������HgCl2����ⶨ��Fe3����________��
(4)�ٿ�ѡ��________(���Լ�)������Һ�к���Fe3��������Fe3����ԭ����________________________________________________________________________(�����ӷ���ʽ��ʾ)��
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������ҡ������Դ�ı��⡣�й�Ҫʵʩ����ǿ��ս�ԣ�ʵ���ɺ���������ǿ�����������롣�����Ѿ���Ϊ�����ҹ����÷�չ����Ҫ���棬��ˮ���ۺϿ����������Ǻ��õ�һ���֣���ˮ�п���ȡ���ֻ���ԭ�ϣ������ǹ�ҵ�϶Ժ�ˮ�ļ����ۺ����õ�ʾ��ͼ������������ͼ��ʾ��
��1��д���١��ڷ�Ӧ�����ӷ���ʽ��
��______________________����______________________��
��2����ҵ�����õ�ⱥ��ʳ��ˮ������������������ȡ���ᣬΪ��������ɫ��ѧ���ʹ������ַ�Ӧ����ȡ��������������ȼ�յİ취���ɱ�������ȼ�ղ���ȫ��Ⱦ��������д��������������ȼ�յ�ʵ������______________________��
��3�������к���Ca������Mg������SO4���������ʣ����ƺ�ɵõ�����NaC1��Һ���������г����Լ���A������ B������������Һ C��̼������Һ������ʱ������������Լ�����ȷ˳����______________��������ţ�
��4������þ�ڿ�����ȼ��ʱ��������MgO�⣬��������Mg3N2���ɡ��ѵ����ʵ����Ľ���þ�ֱ���ڣ�A����������O2���У�B��������̼�����У�C�������С���ȫȼ�պõ��Ĺ������ʵ������ɴ�С��˳����______________��������ţ�
��5������ⱥ��NaCl��Һ���ɵ�����ͨ������������Һ�п��Եõ�NaClO��ij��ѧ��ȤС��̽��NaClO������CO(NH2)2�ķ�Ӧ���ͨ��ʵ�鷢�ֲ����ij�����⣬������ﶼ���ܲ������ѭ�������ʣ���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʯ���顢ʯ�ҵ�(CaCN2)��������(��H2S)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��CS(NH2)2(����)���䲿�ֹ����������£�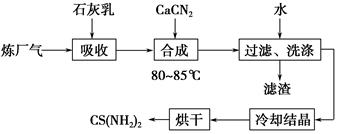
(1)�����£�H2S�������з�Ӧ��2H2S(g)??2H2(g)��S2(g)����ƽ�ⳣ������ʽΪK��________________��
(2)��ʯ��������H2S��ȡCa(HS)2��Ҫ�ڵ����½��У���ԭ����_____________________________________________________��
���˵õ��������������ã���������Ҫ�ɷ���________(�ѧʽ)��
(3)�ϳ������賤ʱ����裬���ڽϸ��¶�(80��85 ��)�½��У���Ŀ����_______________________________________��
Ca(HS)2��CaCN2��ˮ��Һ�кϳ�����Ļ�ѧ����ʽΪ________________________________��
(4)������X�����廥Ϊͬ���칹�壬X����FeCl3��Һ�У���Һ�Ժ�ɫ��X�Ļ�ѧʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ƿ��ɫ��Һ�ı�ǩ���ˣ�ֻ֪�����Ƿֱ������ᡢBaCl2��Һ��Na2CO3��Һ���ס�����λͬѧ����һ���Լ���һ���Լ���ȡ���˳ɹ������õ�һ��ָʾ����_________�����õ�һ������Һ��__________����ͬѧû���κ��Լ�Ҳ����ɹ��ˣ����ļ�������ǣ�ȡ�����Թ��У����Ϊa��b��c��Ȼ��������Һ������ϣ�����a����b�������ݲ�����a����c���а�ɫ������������a��b��c�ֱ���________��________��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��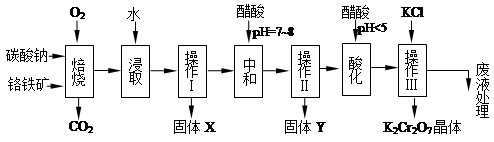
��֪����4FeO��Cr2O3+ 8Na2CO3+ 7O2 8Na2CrO4 + 2 Fe2O3 + 8CO2����
8Na2CrO4 + 2 Fe2O3 + 8CO2����
��Na2CO3 + Al2O3 2NaAlO2 + CO2������ Cr2O72��+ H2O
2NaAlO2 + CO2������ Cr2O72��+ H2O 2CrO42�� + 2H+
2CrO42�� + 2H+
��������ش��������⣺
��1������X����Ҫ����_________����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ��__________����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH<5����Ŀ����_________________________________��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� �����ˡ�_______�����
��4���±���������ʵ��ܽ�����ݣ�����������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl ��K2Cr2O7��+2NaCl���÷�Ӧ����Һ���ܷ�����������_______________��
| ���� | �ܽ��/(g/100gˮ) | ||
| 0��C | 40��C | 80��C | |
| KCl | 28 | 40.1 | 51.3 |
| NaCl | 35.7 | 36.4 | 38 |
| K2Cr2O7 | 4.7 | 26.3 | 73 |
| Na2Cr2O7 | 163 | 215 | 376 |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com