
ij�������Ա�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�����ҩ����������

�ش��������⣺
��1�����������ӣ�����˵����ȷ����________��
| A��1 mol�����������Ժ�2 mol NaOH��Ӧ |
| B��������������Ӧ |
| C���ɷ���ˮ�ⷴӦ |
| D�������巢��ȡ����Ӧ |
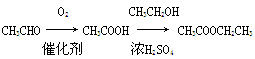

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����15�֣���֪����RCH2COOH��Cl2 RCHClCOOH��HCl��RΪ������
RCHClCOOH��HCl��R������
��R�䣭ONa��R��CHClCOOH R�䣭OCHRCOOH��NaCl��R��R��Ϊ������������ͬҲ���Բ�ͬ��
R�䣭OCHRCOOH��NaCl��R��R��Ϊ������������ͬҲ���Բ�ͬ��
�л���E����Ҫ���л��ϳ��м��壬��ϳɹ�����������ͼ��ʾ��
�л���A����Է�������Ϊ122������̼��������������ֱ�Ϊ78.7%��8.2%��A����ˮȡ����Ӧ�IJ���ֻ��һ�֣�A�ĺ˴Ź���������4�����塣
��1��д��A��C�Ľṹ��ʽ:A________��C_________��
��2����Ӧ������ ��Ӧ��A��B�ķ�Ӧ�У�C2H5OH��������________________��
��3��д����Ӧ�ڵĻ�ѧ����ʽ_________________��
��4��B��ϵͳ������________________��
��5���л���D��ͬ���칹��Fˮ������ữ���ܷ���˫���Ӽ�������Ӧ�γ���Ԫ���л���M����M�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�����ñ�ϩ�ͶԶ��ױ��ϳ��л��߷��Ӳ���W��ת����ϵʾ��ͼ��
��֪����C��������B���Է���������Ӧ��1mol D�������ƿ�����22.4L H2����״���£���
��
��1��A�Ľṹ��ʽ��________��
��2��C�����������ŵ�����Ϊ ��
��3��B��C��Ӧ����Ϊ________��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
A��B��_________________________��
D��E��W��_________________________��
��5��д����������������һ��E��ͬ���칹�壺___________________��
����FeCl3��Һ����ɫ �ڿɷ���������Ӧ �ۿ���NaHCO3��Һ����CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����廯����A������ֻ��һ��ȡ����,�����ķ�Ӧ��ͼ��ʾ,B��F��G�ķ���ʽ�ֱ�ΪC2H4O2��H2C2O4��C6H12O,H��ʹ��ˮ��ɫ��
(1)A�Ľṹ��ʽ ,H�ļ���ʽ ��
(2)B�еĹ���������Ϊ ,�ݵķ�Ӧ����Ϊ ��
(3)C��һ���������γɸ߷��ӻ�����Ļ�ѧ����ʽ ��
(4)����G��F��Ӧ�Ļ�ѧ����ʽ ��
(5)G��ͬ���칹�����ܷ���������Ӧ�Ĺ��� ��,���к˴Ź���������������������л���ṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��
ˮ����EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�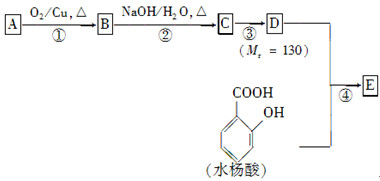
��ش��������⣺
(1)һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ________���ṹ������ʾAֻ��һ������A������Ϊ________��
(2)B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ__________________________��
(3)C��________�ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���________��
(4)�ڢ۲��ķ�Ӧ����Ϊ________��D���������ŵ�����Ϊ________��
(5)д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ��__________________________��
a����������6��̼ԭ����һ��ֱ���ϣ�
b�����������������Ű���ˮ������еĹ����š�
(6)�ڢܲ��ķ�Ӧ����Ϊ________��д��E�Ľṹ��ʽ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ۣ�3���ǻ���������PHB������������ɽ������ϵȡ�PHB����3���ǻ�����[CH3CH��OH��CH2COOH]���Ӿۺ϶��ɡ��ϳɾۣ�3���ǻ���������;���ܶ࣬������һ��;���ĸ������١���ȾС��ԭ�������ʸߣ���ϳ�·�����£�
��֪�� 

��ش��������⡣
��1��д��C�к��еĹ����ŵ����ƣ�____________��
��2������ת�����������ڼӳɷ�Ӧ����________������ţ���
��3����Ӧ�ڷ�����ɫ��ѧ˼�루̼ԭ�ӵ���Ч������Ϊ100%������A�Ľṹ��ʽΪ__________________��
��4��д����Ӧ�ܵĻ�ѧ����ʽ��_____________________________________
_______________________________________________________________��
��5��д����C��Ϊͬ���칹�壬�ܷ���������Ӧ���Һ˴Ź����������������շ���л���Ľṹ��ʽ��____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ᱽ������������ϩ������һ�ֻ���ԭ��ͨ�����кϳɷ�Ӧ�Ƶã����Ի����������Ͼ����������ã��ʳ����ڻ�ױƷ��ҵ��ʳƷ��ҵ�����Ӽ�����ƺϳɷ������£�

��1��д���������ʵĽṹ��ʽ��A ��C�� ��F��
��2�� �жϻ�ѧ��Ӧ���ͣ�A��B ��E��F
��3��D�кܶ�ͬ���칹�壬����������һȡ�����ṹ��ͬ���칹������������������Ľṹ��ʽ��
��д����������ͬ���칹��Ľṹ��ʽ�� ��
��4��д��C��D�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ϩ�����ںϳ�ɱ�������߳��ũҩ������ʽΪC3H5Br2Cl����Ӧ�ù㷺��DAP��֬��
��֪���봼�ɷ���������������Ӧ��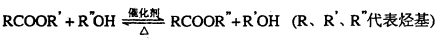
��1��ũҩC3H5Br2Cl������ÿ��̼ԭ���Ͼ�����±ԭ�ӡ�
A�Ľṹ��ʽ��__________________��A�����������ŵ�������____________________��
��ϩ��A�ķ�Ӧ������_______________��A��C3H5Br2CI�ķ�Ӧ������_____________��
��2��Aˮ��ɵõ�D����ˮ�ⷴӦ�Ļ�ѧ����ʽΪ��
_______________________________________________________________________.
��3��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����S�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%. S�Ľṹ��ʽΪ_________________________________��
��4������˵����ȷ����______________������ĸ���ţ���
a��C�ܷ����ۺϷ�Ӧ����ԭ��Ӧ��������Ӧ
b��C����2������������ͬ���칹����4��
c��D������IJ�����B������ͬ����Է�������
d��E���з�����ζ���������Ҵ�
��5��E��ˮ����ᆳ�������յõ��״���B�����߾���ѭ��������DAP��֬���Ʊ������н��״���H����IJ���������__________________��
��6��F�ķ���ʽΪC10H10O4. ��DAP����Ϊ���Ķ�Ԫȡ���������ȡ���������ڶ�λ���õ��屽���ϵ�һ��ȡ����ֻ�����֡�����D��F��Ӧ����DAP����Ļ�ѧ����ʽΪ��
_______________________________________________________________________.
��7��ʵ������2-�����Ʊ���ϩʱ������������SO2�� CO2��ˮ������ijͬѧ�������Լ�
�������������壬�������ͨ���Լ���˳����_______________������ţ���
�ٱ���Na2SO3��Һ������KMnO4��Һ ��ʯ��ˮ ����ˮCuSO4��Ʒ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����CHO+(C6H5)3P=CH��R ��CH=CH��R + (C6H5)3P=O��R����ԭ�ӻ�ԭ���ţ�W��һ���л��ϳ��м��壬�ṹ��ʽΪ��HOOC��CH=CH��CH=CH��COOH����ϳɷ������£�
���У� �ֱ����һ���л���ϳɹ�������������ͷ�Ӧ��������ȥ��
�ֱ����һ���л���ϳɹ�������������ͷ�Ӧ��������ȥ��
X��W��һ�������·�Ӧ����������N��N����Է�������Ϊ168��
��ش��������⣺
��1��W�ܷ�����Ӧ�������� ������д��ĸ��ţ�
| A��ȡ����Ӧ | B��ˮ�ⷴӦ | C��������Ӧ | D���ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com