
����Ŀ���ñ�Ũ�ȵ�NaOH��Һ���ζ�δ֪Ũ�ȵ����ᣬ���в����л�ʹ����ⶨŨ�ȱ�ʵ��Ũ��ƫ�ߵ��ǣ�������
�ټ�ʽ�ζ���������ˮϴ����δ�ñ���Һ��ϴ������ƿ������������ˮ��ʵ��ʱû�к�ɴ�������ȡδ֪Ũ���������ʽ�ζ���������ˮϴ����δ�ô���������ϴ���ܵζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ���ݵζ������ʱ�����Ӷ�����
A. �٢� B. �ڢ� C. �٢� D. �ܢ�
���𰸡�A
���������ټ�ʽ�ζ���������ˮϴ����δ�ñ���Һ��ϴ���ζ����ڼ�Һ��Ũ�Ⱦͻ��С�����Ի�������ļ ![]() ����ʹ
����ʹ![]() ƫ�ߣ�����ȷ��
ƫ�ߣ�����ȷ��
����ƿ������������ˮ��ʵ��ʱû�к�ɴ������������ڵζ����Ӧ����Ӱ�죬ԭ��������ƿ�м������ˮ����Ӱ��μӵ�����������Һ��������ڴ���
��ȡδ֪Ũ���������ʽ�ζ���������ˮϴ����δ�ô���������ϴ����ȡ�������ᱻˮϡ�ͣ�Ũ��ƫС�����µμӵ�����������Һ���ƫС�����յζ����ƫС���۴���
�ܵζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ�����ʽ�ζ��ܵĶ��������μӽ���ƿ�������ԭ���ļ������ݵ���������Ե���![]() ����ʹ
����ʹ![]() ƫ�ߣ�����ȷ��
ƫ�ߣ�����ȷ��
�ݵζ������ʱ�����Ӷ����������ƫС�����Ե���![]() ��С��ʹ
��С��ʹ![]() ƫ�ͣ��ݴ���
ƫ�ͣ��ݴ���
����ƫ�ߵ��Ǣ٢ܣ�ѡ��A��ȷ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӡ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ���⡣
(1)�õ绡���ϳɵĴ�������̼�ܳ����д�����̼�����������ʣ������ֿ������������������ᴿ������ɸ÷�Ӧ�Ļ�ѧ����ʽ��
___ C+ ___ KMnO4+ H2SO4 �� ____CO2��+ ____MnSO4 + ____K2SO4+
(2)����ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�ӦCO(g)��H2O(g)![]() CO2(g)��H2(g)���õ����¶������ݣ�
CO2(g)��H2(g)���õ����¶������ݣ�
ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
CO | H2O | H2 | CO | |||
1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
��ʵ��1����CO2��ʾ�Ļ�ѧ��Ӧ����Ϊ__________������С������λ������ͬ����
��ʵ��2������ƽ�ⳣ��K=_________���÷�ӦΪ _____��������š����ȷ�Ӧ��
(3)��֪�ڳ��³�ѹ�£�
�� 2CH3OH(l)��3O2(g) �� 2CO2(g)��4H2O(g) ��H �� ��1275.6 kJ��mol
�� 2CO (g)+ O2(g) �� 2CO2(g) ��H �� ��566.0 kJ��mol
�� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol
д���״�����ȫȼ������һ����̼����̬ˮ���Ȼ�ѧ����ʽ��_____________��
(4)ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�á�

�ٸõ�ظ����ĵ缫��ӦΪ��_______________��
�ڸõ�ع���ʱ����Һ�е�OH����______���ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϸ������������ˮ����NO2��SO2�Ļ�ѧ��Ӧ�ǵ�ǰ�����ڼ������ε���Ҫ����·����ijʵ��С��������ijɷֽ�������֤�����ⶨ������SO2�ĺ�����̽��H2SO3�IJ������ʡ�
�ش�����������
��1���������ij��PM2.5��������Ҫ�ɷ֣��ռ�һ�������������������֤��
ȡһ�����������������Թ��У�����������ˮ�ܽ⣬����Һ�ֳ�����ʢ���Թ���:
�������� | ʵ������ | ���� |
��������һ��_______ | �а�ɫ�������� | ֤�����������к���SO42- |
������һ����_____�������Ӽг�ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | ���Թ��������ݲ�����___________________ | ֤�����������к���NH4+���ۺ�����ʵ��,����˵���������ݿ����к���(NH4)2SO4 |
��2������ͼ��ʾ����װ�òⶨ������SO2�ĺ�����
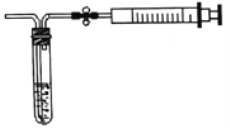
���ⶨԭ����SO2ͨ���ĵ�����Һ�У�ʹ��Һ����ɫ��Ϊ��ɫ����Ӧ�Ļ�ѧ����ʽΪ______��
���ⶨ��������ij���㣬��ȡ5.0mL5.0��10-4mol/L�ĵ���Һ��ע��ͼ�е��Թ��У���2-3�ε���ָʾ������ʱ��Һ����ɫ����ͼ��װ�����Ӻ�����������ֹˮ�п��ƣ����г�����ȡ��ע�����������ظ�����ֱ����Һ����ɫȫ���ʾ�Ϊֹ������ȡ����8000.0mL�����øü��������SO2�ĺ���Ϊ_____mg/L��
��3��̽��H2SO3�IJ������ʡ�
ѡ�������װ�ú�ҩƷ̽��H2SO3��HClO������ǿ��
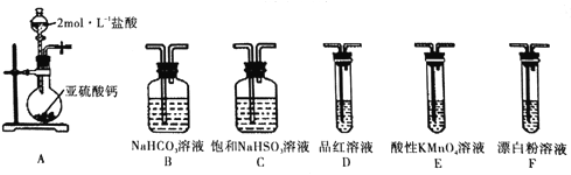
�ټ�ͬѧ��Ϊ����A��C��F��β��������˳������װ�ã�����֤��H2SO3��HClO������ǿ������ͬѧ��Ϊ�÷�������������������_________________��
�ڱ�ͬѧ���ü�ӷ�֤����ʵ�鷽��Ϊ������A��C____(����ĸ) ��β������˳������װ�ã�����װ��C��������___________��֤��H2SO3������ǿ��HClO�����Ե�ʵ��������_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����Ϊ�������й������ĺ�������������Ϳ��CuI����ֽ��������ֽ�Ƿ��ɫ����ɫ�����仯����ȥ��ʱ�����жϿ����еĺ��������䷴ӦΪ4CuI��Hg===Cu2HgI4��2Cu(��Ӧǰ���I��Ϊ��1���Ҳ����еĹ�Ԫ��Ϊ��2��)��
(1)������Ӧ����Cu2HgI4�У�ͭԪ����______�ۡ�
(2)���Ϸ�Ӧ�е�������Ϊ________����ԭ��Ϊ________������������________����ԭ������________������2 mol CuI���뷴Ӧʱ��ת�Ƶ���________ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�ռ���Ʒ�к����������������õĿ��������ʣ�Ϊ�˲ⶨ�䴿�ȣ��������µζ�������
A����250 mL����ƿ������250 mL�ռ���Һ
B������Һ�������ʽ�ζ�������ȡ25.00 mL �ռ���Һ����ƿ�в��Ӽ��η�ָ̪ʾ��
C������ƽ��ȷ��ȡ�ռ���Ʒw g�����ձ��м�����ˮ�ܽ�
D�������ʵ���Ũ��Ϊm mol��L��1�ı�H2SO4��Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ�̶�V1 mL
E������ƿ�µ�һ�Ű�ֽ���ζ����յ㣬��¼�յ�̶�ΪV2 mL
������������⣺
��1����ȷ�IJ�������������д��ĸ�� ____��____��____��D��____��
��2������D��Һ��Ӧ������_______________�����첿��Ӧ________________��
��3�����²�������������ռ���ҺŨ��ƫ�͵�����____��
A����ʽ�ζ���δ�ô�װ��Һ��ϴ B����ʽ�ζ���δ�ô�װ��Һ��ϴ
C����ƿδ�ô�װ��Һ��ϴ D���ڵζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ
��4�����ռ���Ʒ�Ĵ��ȼ���ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������һ����Ҫ�Ļ���ԭ�ϣ���ش���������:
I.�������(K2FeO4)��Һ���Ϻ�ɫ�������м�������������ƺ���Һ�Ϻ�ɫ����ȥ�������ֺ��ɫ��������д���÷�Ӧ�����ӷ���ʽ__________��
II.ʵ����ģ����ͼ��ʾ�����Ʊ���������:

��֪:�����������У����Ʒ�Ӧ�¶���35~60����������Ҫ������Ӧ��
C6H12O6+12HNO3=3HOOC-COOH+9NO2��+3NO��+9H2O
������������Һ����NO��NO2������Ӧ:
NO+NO2+2NaOH=2NaNO2+H2O��2NO2+2NaOH=NaNO3+NaNO2+H2O

(1)ľм����Ҫ�ɷ�����ά�أ������֪��Ϣ�٣�����Ϊ��ľм�м�ϡ�����������______�����������з�Ӧ�¶Ȳ��˸���60�棬ԭ����______________��
(2)�����κδ��������˹��̽��У�����������Һ���պ����Һ�г���OH-������������ӣ�����һ����NO2-��NO2-����һ�������ӵ����ʵ���֮��Ϊ________��
(3)װ��B�����Ʊ�NaNO2��ʢװ���Լ���NaOH(aq)�⣬��������______(����ĸ)��
a.NaCl(aq) b.Na2CO3(aq) c.NaNO3(aq)
III.�ⶨ��Ʒ����:
��ʵ�鲽�衿��ȷ����ag��Ʒ���200mL��Һ���ڴӲ�������Ƶ���Һ����ȡ20.00mL������ƿ�У�����cmol/L����KMnO4��Һ�ζ����յ㣻���ظ����ϲ���3�Σ���������KMnO4��Һ��ƽ�����ΪVmL
(1)��ƿ�з�����Ӧ�����ӷ���ʽΪ_____________���ﵽ�ζ��յ��������_________��
(2)��Ʒ��NaNO2�Ĵ���Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�����о������У��ܲ���л�����Է��������������ǣ�������
A. ���������
B. Ԫ�ط�����
C. ������
D. �˴Ź�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼ��ʾϸ�������л������ɡ��������﹦�ܣ������ش��������⣺
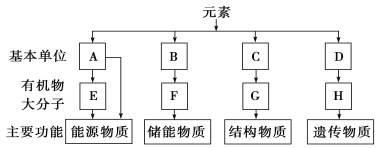
(1)A��ָ________��E�ڶ���ϸ������ָ________����ֲ��ϸ������Ҫ��ָ________��
(2)F��ָ________��������B(֬�������)�γɵģ�����֮�⣬֬�ʻ�����________ ��________��
(3)C��ָ________��ͨʽ��________��C�γ�G���̵ķ�Ӧ��________��
(4)D��ָ________��H��ָ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.5mol Al2��SO4��3�к���Al3+����ĿԼ�� ������
A.3.01��1023
B.6.02��1023
C.0.5
D.1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com