
AԪ�ص�һ�ֵ�����һ����Ҫ�İ뵼����ϣ���AԪ�ص�һ�ֻ�����C��������������ܵ��ִ�ͨѶ���ϡ������ά��C���ռӦ���ɺ�AԪ�صĻ�����D��
��1����Ԫ�����ڱ��У�Aλ�ڵ�________�壬��Aͬ�嵫���ԭ��������AС��Ԫ��B��ԭ�ӽṹʾ��ͼΪ________��A��B��ԭ�ӵĵ��Ӳ�ṹ�ϵ���ͬ����_______________________��
��2������C������ѧ��Ӧ������______ �������ƣ�����Ӧ�Ļ�ѧ����ʽ��_______��
��3����C�봿���ϸ�������ʱҲ������ѧ��Ӧ����D��ͬʱ������B�����������E����ȫ����E��ȫ����D��������ˮ�л�Ϻ��ַ�����ѧ��Ӧ���ɺ�A�Ļ�����F��
��д������D��F�Ļ�ѧ����ʽ��______________________________________��___________________________________________________________________��
��Ҫ��NaOH�����ۻ������������в���ѡ�õ���_________________________��
A.��ͨ�������� B.ʯӢ��������
C.���������� D.������
��4��100 g C��ʯ��ʯ�Ļ�����ַ�Ӧ�����ɵ������ڱ�״���µ����Ϊ11.2 L��100 g�������ʯ��ʯ������������________��
��1����A 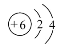 ��������4�����ӣ����ڲ����2������
��������4�����ӣ����ڲ����2������
��2������� SiO2+4HF=SiF4��+2H2O
��3����SiO2+Na2CO3 Na2SiO3+CO2��
Na2SiO3+CO2��
Na2SiO3+CO2+H2O=Na2CO3+H2SiO3��
��A��B��C
��4��50%
����������1��AԪ�ص��ʿ����뵼����ϣ���AԪ�ص�ij��������������ά��ԭ�ϣ���֪AΪ��Ԫ�أ��ȹ����ԭ������С��ͬ��Ԫ��Ϊ̼��
��2��CΪSiO2������SiO2��Ӧ����ֻ������ᡣ
��3��SiO2��NaOH��Ӧ����Na2SiO3��H2O���ʺ�SiO2�IJ��ϣ���ͨ������ʯӢ�������Լ�Al2O3�ȶ�����NaOH�ڸ����·�Ӧ���ʲ��������ϲ��ʵ������ۻ�NaOH��
��4�����ݷ�ӦSiO2+CaCO3 CaSiO3+CO2����
CaSiO3+CO2����
CaCO3 CaO+CO2����֪������SiO2��CaCO3�Ƿ�ǡ����ȫ��Ӧ�����й�ϵʽCaCO3��CO2���ڣ���n��CaCO3��=n��CO2��=0.5 mol��m��CaCO3��=50 g��
CaO+CO2����֪������SiO2��CaCO3�Ƿ�ǡ����ȫ��Ӧ�����й�ϵʽCaCO3��CO2���ڣ���n��CaCO3��=n��CO2��=0.5 mol��m��CaCO3��=50 g��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 5-3��ѧ����ϰ���������棩 ���ͣ�ѡ����
���Ȼ�����S2Cl2���ǹ㷺������ҵ����������ӽṹ��ͼ��ʾ�������£�S2Cl2��ˮ��ˮ�⣬��������ʹƷ����ɫ�����塣����˵��������ǣ� ��

A.S2Cl2Ϊ���ۻ�����
B.S2Cl2ֻ���м��Թ��ۼ�
C.S2Cl2��ˮ��ӦʱS��S����S��Cl��������
D.S2Cl2������S��S����S��Cl����ͨ�����õ��ӶԵ�������γɵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-4��������Ҫ��������ϰ���������棩 ���ͣ�ѡ����
��֪������������ˮ�����������л��ܼ�CCl4������װ���в�������������β�����յ��ǣ� ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-2�����ں�ˮ�е�Ԫ��-����ϰ���������棩 ���ͣ������
�����£�����A��B��C�ֱ�Ϊ���塢����ɫ���塢��ɫ���塣�ں��ʵķ�Ӧ�����£����ǿ��������ͼ���з�Ӧ����֪E��Һ����ɫ�ġ���ش�

��1��A��_______��B��_______��C��_______ �����ѧʽ����
��2����Ӧ�ٵĻ�ѧ����ʽΪ�� ___________________________________��
��3����Ӧ�۵����ӷ���ʽΪ�� _________________________________________��
��4����Ӧ�ܵ����ӷ���ʽΪ��___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-2�����ں�ˮ�е�Ԫ��-����ϰ���������棩 ���ͣ�ѡ����
���ж��ȼ��仯�����˵����ȷ���ǣ� ��
A������������������ȼ�տ������Ȼ�����
B����pH��ֽ�����ˮ��pH=4
C���廯����Һ�м�������������ˮ���ټ����������Ȼ�̼�����ú��ϲ���ɫ��dz���²���ɫ��Ⱥ�ɫ
D����Ca��ClO��2+CO2+H2O=CaCO3��+2HClO���Ƴ�Ca��ClO��2+SO2+H2O=CaSO3��+2HClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-1���ǽ������ϵ�����-����ϰ���������棩 ���ͣ�ѡ����
����˵����ȷ���ǣ� ��
A.CO��NO��NO2���Ǵ�����Ⱦ���壬�ڿ����ж����ȶ�����
B.�����ש�е��������ɷ֣����ש��ĩ�м������ᣬ��ַ�Ӧ��ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ2��3�μ���
C.�ڴֹ����ȡ�У�2C+SiO2 Si+2CO���豻��ԭ�����Թ�ķǽ����Ա�̼ǿ
Si+2CO���豻��ԭ�����Թ�ķǽ����Ա�̼ǿ
D.��SiO2��ȡ���ᣬӦ��ʹ��������������������Һ��Ӧ��Ȼ��ͨCO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-3��������Ҫ��������ϰ���������棩 ���ͣ������
�����������壨FeSO4��7H2O����ҽҩ������Ѫ����ij����С���ͬѧ���ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�

��ش��������⣺
��1��֤���������Һ�к���Fe2+�ķ����ǣ�ȡ�����ȵμ�KSCN��Һ���ٵμ�_______���ù��̵�����Ϊ��__________________________________________��
��2������ڼ������H2O2��Ŀ���ǣ�____________________________________��
��3��������з�Ӧ�����ӷ���ʽ�ǣ�_____________________________________��
��4���������һϵ�д����IJ������裺���ˡ�_______�����ա�_______��������
��5����ʵ����������ģ���ÿƬ��Ѫ���к���Ԫ�ص�����Ϊ_______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-2��������Ҫ��������ϰ���������棩 ���ͣ�ѡ����
�ں�1 mol KAl��SO4��2����Һ����μ���2 mol Ba��OH��2��Һ�������й�˵������ȷ���ǣ� ��
A�������ɳ����������ȫ���ܽ�
B������Һ��Ba2+ȫ����������ʱ�������������ʵ������
C�����õ��ij�����BaSO4
D�����ij���Ϊ2 mol BaSO4��1 mol Al��OH��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 2-2���ӷ�Ӧ��ϰ���������棩 ���ͣ�ѡ����
�������Һ��Al3+��Cu2+��Ba2+��Ag+��һ�����������Է��룬�����Լ���Cl- �� ��OH-��CO2 ��
��OH-��CO2 �� ����ȷ˳���ǣ� ��
����ȷ˳���ǣ� ��
A.�٢ڢۢ� B.�ۢݢ٢� C.�ڢ٢ۢ� D.�٢ݢۢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com