
���Ϲ����к���ƻ���ᣬƻ���ᾭ�ۺ����ɾ�ƻ���ᡣ��֪��
a��0.1 molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48 L CO2����״������
b�� ƻ������ˮ������ʹ��ˮ��ɫ�IJ��
c��RCH2Br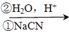 RCH2COOH ��
RCH2COOH ��
d��RC��CH+R��CHO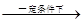 RC��CCH-OH
RC��CCH-OH
R��

��ش�
��1��д��B�Ľṹ��ʽ ��
��2��д��ƻ�������������ŵ����� �� Fת����ƻ������ܷ����ķ�Ӧ���� ��
��3��д����ƻ���������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ ��
��4��д��F������������Һ��Ӧ�Ļ�ѧ����ʽ ��
��5��д��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��6����ƻ����������õ����������ԣ�����Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽ ��
��1�� HOOCCH2CH2COOH 1��
��2���ǻ����Ȼ�2�֣���1��
������ȡ����ˮ�� д��1����1�֣����2�֣��д���1�֣�ֱ��0��
��3�� ��1��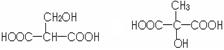
��4��OHCCH2CHClCHO +4 Ag��NH3��2OH  NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O 2��
NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O 2��
��5��HOOCCH2CHClCOOH + 3NaOH 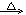 NaOOCCH2CH(OH)COONa + NaCl + 2H2O 2��
NaOOCCH2CH(OH)COONa + NaCl + 2H2O 2��
��4��5�����нṹ��ʽд����ȱ�ٲ��ﲻ���֣�δ��ƽ��1�֣�
��6��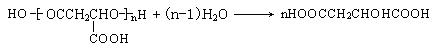
��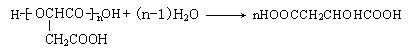
1�֣��ṹ��ʽд����ȱ�ٲ��ﲻ���֣��� ��д�ɡ�
��д�ɡ� ���Ų��۷֣�
���Ų��۷֣�
���������������1��������������������Ϣ������B�к���-COOH���ṹ��ʽΪHOOCCH2CH2COOH��
��2��ƻ�������������ŵ�����Ϊ�ǻ����Ȼ���Fת����ƻ������ܷ����ķ�Ӧ����Ϊ������ȡ����ˮ�⡣
��3����ƻ���������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��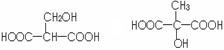 ��
��
��4��F������������Һ��Ӧ�Ļ�ѧ����ʽΪOHCCH2CHClCHO +4 Ag��NH3��2OH 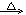 NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O��
NH4OOCCH2CHClCOONH4 +4 Ag��+6NH3+2H2O��
��5��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪHOOCCH2CHClCOOH + 3NaOH 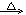 NaOOCCH2CH(OH)COONa + NaCl + 2H2O��
NaOOCCH2CH(OH)COONa + NaCl + 2H2O��
��6������������ˮ��Ļ�ѧ����ʽΪ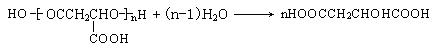 ��
��
���㣺ƻ�����Ӧ�� �л��ƶ�
���������⿼�����ƻ�����Ӧ�ú��л��ƶϵ����֪ʶ����Ŀ�Ѷȴ����ú�������Ϣ�ǽ���Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| Cl2 |
| ���� |

| ��NaCN |
| ��H2O��H+ |

| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |


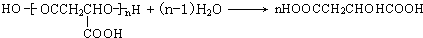
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

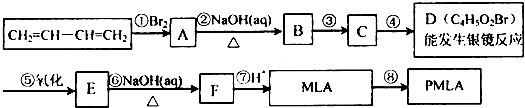
(1)д���������ʵĽṹ��ʽ��A____________��D____________��
(2)ָ����Ӧ���ͣ���____________����____________��
(3)д��������MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��____________________��
(4)д��E��Fת���Ļ�ѧ����ʽ��______________________________________________��
(5)����ת����ϵ�в���ۺܵ͢�˳���ܷ�ߵ���____________(��ܡ����ܡ�)��˵�����ɣ�_____________________________________________________________________��
(6)PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȫ������Ϲ����к���ƻ���ᣨMLA)�������ʽΪC4H6O5��0.1 molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48 L CO2����״������ƻ������ˮ������ʹ��ˮ��ɫ�IJ��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA����
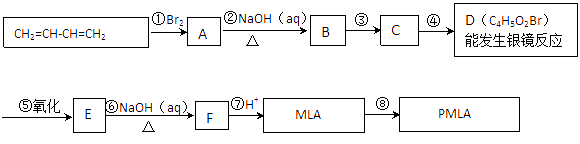
��1��д���������ʵĽṹ��ʽ��A ��D ��
��2��ָ����Ӧ���ͣ��� �� ��
��3��д��������MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ�� ��
��4��д��E��Fת���Ļ�ѧ����ʽ ��
��5������ת����ϵ�в���ۺܵ͢�˳���ܷ�ߵ��� ����ܡ����ܡ���˵�����ɣ� ��
��6��PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�ൺ�и�����������ģ����ѧ�Ծ��������棩 ���ͣ��ƶ���
���Ϲ����к���ƻ���ᣨMLA���������ʽΪC4H6O5��0.1molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48LCO2����״������ƻ������ˮ������ʹ��ˮ��ɫ�IJ��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA����
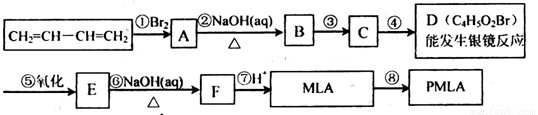
��1��д���������ʵĽṹ��ʽ��B______��D______��
��2��MLA�ĺ˴Ź���������____��塣��MLA������ͬ�����ŵ�ͬ���칹����_____�֡�
��3��д��E��Fת���Ļ�ѧ����ʽ______________��
��4������ת����ϵ�в���ۺܵ͢�˳���ܷ�ߵ�______����ܡ����ܡ���˵�����ɣ�______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com