
ΓΨΧβΡΩΓΩA(C3H6) «Μυ±Ψ”–ΜζΜ·ΙΛ‘≠ΝœΓΘ”…A÷Τ±ΗΨέΚœΈοCΚΆ![]() ΒΡΚœ≥…¬ΖœΏ(≤ΩΖ÷Ζ¥”ΠΧθΦ଑»Ξ)»γΆΦΥυ ΨΘΚ
ΒΡΚœ≥…¬ΖœΏ(≤ΩΖ÷Ζ¥”ΠΧθΦ଑»Ξ)»γΆΦΥυ ΨΘΚ
“―÷ΣΘΚ
ΔΌ![]() +
+![]()
![]()
![]()
ΔΎRΓΣCΓ‘N![]() RΓΣCOOH
RΓΣCOOH
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)DΒΡΟϊ≥Τ «___________Θ§BΚ§”–ΒΡΚ§―θΙΌΡήΆ≈ΒΡΟϊ≥Τ «__________ΓΘ
(2)CΒΡΫαΙΙΦρ ΫΈΣ_____________Θ§DΓζEΒΡΖ¥”Πάύ–ΆΈΣ ____________ΓΘ
(3)EΓζFΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___________ΓΘ
(4)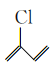 ÷–ΉνΕύ”–_____Ηω‘≠Ή”Ι≤ΤΫΟφΘ§
÷–ΉνΕύ”–_____Ηω‘≠Ή”Ι≤ΤΫΟφΘ§![]() ΖΔ…ζΥθΨέΖ¥”Π…ζ≥…”–ΜζΈοΒΡΫαΙΙΦρ ΫΈΣ__________ΓΘ
ΖΔ…ζΥθΨέΖ¥”Π…ζ≥…”–ΜζΈοΒΡΫαΙΙΦρ ΫΈΣ__________ΓΘ
(5)BΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§”κBΨΏ”–œύΆ§ΒΡΙΌΡήΆ≈«“ΡήΖΔ…ζ“χΨΒΖ¥”ΠΒΡΙ≤”–_______÷÷(≤ΜΩΦ¬«ΝΔΧε“λΙΙ)ΘΜΤδ÷–ΚΥ¥≈Ι≤’ώ«βΤΉΈΣ3ΉιΖεΘ§«“ΖεΟφΜΐ÷°±»ΈΣ6:1:1ΒΡ «__________(–¥ΫαΙΙΦρ Ϋ)ΓΘ
(6)ΫαΚœΧβΗχ–≈œΔΘ§“‘““œ©ΓΔHBrΈΣΤπ Φ‘≠Νœ÷Τ±Η±ϊΥαΘ§…ηΦΤΚœ≥…¬ΖœΏ(ΤδΥϊ ‘ΦΝ»Έ―Γ)________ΓΘ
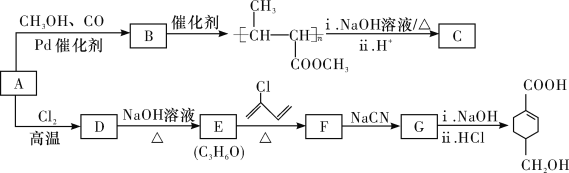
ΓΨ¥πΑΗΓΩ3-¬»±ϊœ© θΞΜυ 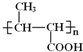 »Γ¥ζΖ¥”ΠΜρΥ°ΫβΖ¥”Π
»Γ¥ζΖ¥”ΠΜρΥ°ΫβΖ¥”Π  10
10 ![]() ΓΘ 8
ΓΘ 8 ![]() C H2=C H2
C H2=C H2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
ΓΨΫβΈωΓΩ
BΖΔ…ζΦ”ΨέΖ¥”Π…ζ≥…ΨέΕΓœ©ΥαΦΉθΞΘ§‘ρBΫαΙΙΦρ ΫΈΣCH3CH=CHCOOCH3Θ§AΈΣC3H6Θ§AΖΔ…ζΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…BΘ§‘ρAΫαΙΙΦρ ΫΈΣCH2=CHCH3Θ§ΨέΕΓœ©ΥαΦΉθΞΖΔ…ζΥ°ΫβΖ¥”Π»ΜΚσΥαΜ·ΒΟΒΫΨέΚœΈοCΘ§CΫαΙΙΦρ ΫΈΣ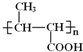 ΘΜAΖΔ…ζΖ¥”Π…ζ≥…DΘ§DΖΔ…ζΥ°ΫβΖ¥”Π…ζ≥…EΘ§EΡήΖΔ…ζΧβΗχ–≈œΔΒΡΦ”≥…Ζ¥”ΠΘ§ΫαΚœEΖ÷Ή” Ϋ÷ΣΘ§EΫαΙΙΦρ ΫΈΣCH2=CHCH2OHΓΔDΫαΙΙΦρ ΫΈΣCH2=CHCH2ClΘ§EΚΆ2-¬»-1Θ§3-ΕΓΕΰœ©ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…FΘ§FΫαΙΙΦρ ΫΈΣ
ΘΜAΖΔ…ζΖ¥”Π…ζ≥…DΘ§DΖΔ…ζΥ°ΫβΖ¥”Π…ζ≥…EΘ§EΡήΖΔ…ζΧβΗχ–≈œΔΒΡΦ”≥…Ζ¥”ΠΘ§ΫαΚœEΖ÷Ή” Ϋ÷ΣΘ§EΫαΙΙΦρ ΫΈΣCH2=CHCH2OHΓΔDΫαΙΙΦρ ΫΈΣCH2=CHCH2ClΘ§EΚΆ2-¬»-1Θ§3-ΕΓΕΰœ©ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…FΘ§FΫαΙΙΦρ ΫΈΣ![]() Θ§FΖΔ…ζ»Γ¥ζΖ¥”Π…ζ≥…GΘ§GΖΔ…ζ–≈œΔ÷–Ζ¥”ΠΒΟΒΫ
Θ§FΖΔ…ζ»Γ¥ζΖ¥”Π…ζ≥…GΘ§GΖΔ…ζ–≈œΔ÷–Ζ¥”ΠΒΟΒΫ Θ§‘ρGΫαΙΙΦρ ΫΈΣ
Θ§‘ρGΫαΙΙΦρ ΫΈΣ![]() ΘΜ
ΘΜ
Θ®6Θ©CH2=CH2ΚΆHBrΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…CH3CH2BrΘ§CH3CH2BrΚΆNaCNΖΔ…ζ»Γ¥ζΖ¥”Π…ζ≥…CH3CH2CNΘ§CH3CH2CN‘ΎΦν–‘ΧθΦΰœ¬ΖΔ…ζΥ°ΫβΖ¥”Π»ΜΚσΥαΜ·ΒΟΒΫCH3CH2COOHΘ§Ψί¥ΥΖ÷ΈωΫβ¥πΓΘ
1. ”…Ζ÷ΈωΩ…÷ΣDΫαΙΙΦρ ΫΈΣCH2=CHCH2ClΘ§ΤδΟϊ≥Τ «3-¬»±ϊœ©Θ§BΫαΙΙΦρ ΫΈΣCH3CH=CHCOOCH3Θ§B÷–Κ§―θΙΌΡήΆ≈Οϊ≥Τ «θΞΜυΓΘ
Ι ¥πΑΗΈΣΘΚ3-¬»±ϊœ©ΘΜθΞΜυΓΘ
2. ”…Ζ÷ΈωΩ…÷ΣCΫαΙΙΦρ ΫΈΣ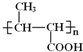 Θ§DΖΔ…ζ»Γ¥ζΖ¥”ΠΜρΥ°ΫβΖ¥”Π…ζ≥…EΘΜ
Θ§DΖΔ…ζ»Γ¥ζΖ¥”ΠΜρΥ°ΫβΖ¥”Π…ζ≥…EΘΜ
Ι ¥πΑΗΈΣΘΚ 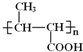 ΘΜ»Γ¥ζΖ¥”ΠΜρΥ°ΫβΖ¥”ΠΘΜ
ΘΜ»Γ¥ζΖ¥”ΠΜρΥ°ΫβΖ¥”ΠΘΜ
3.EΫαΙΙΦρ ΫΈΣCH2=CHCH2OHΓΔFΫαΙΙΦρ ΫΈΣ![]() Θ§EΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…FΘ§ΗΟΖ¥”ΠΖΫ≥Χ ΫΈΣ
Θ§EΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…FΘ§ΗΟΖ¥”ΠΖΫ≥Χ ΫΈΣ ΓΘ
ΓΘ
Ι ¥πΑΗΈΣΘΚ ΓΘ
ΓΘ
4. ΗΟΖ÷Ή”÷–Κ§”–10Ηω‘≠Ή”Θ§ΗυΨί““œ©ΫαΙΙΧΊΒψ÷ΣΘ§ΗΟΖ÷Ή”÷–Υυ”–‘≠Ή”Ι≤ΤΫΟφΘΜΗΟ”–ΜζΈοΖΔ…ζΥθΨέΖ¥”Π≤ζΈοΫαΙΙΦρ ΫΈΣ![]() ΓΘ
ΓΘ
Ι ¥πΑΗΈΣΘΚ10ΘΜ![]() ΓΘ
ΓΘ
5. BΫαΙΙΦρ ΫΈΣCH3CH=CHCOOCH3Θ§BΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§”κBΨΏ”–œύΆ§ΒΡΙΌΡήΆ≈«“ΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§ΥΒΟςΚ§”–ΧΦΧΦΥΪΦϋΚΆθΞΜυΓΔ»©ΜυΘ§ΈΣΦΉΥαθΞΘ§ΖϊΚœΧθΦΰΒΡΆ§Ζ÷“λΙΙΧε”–HCOOCH=CHCH2CH3 HCOOCH2CH=CHCH3 HCOOCH2CH2CH=CH2 HCOOC(C H3)=CHCH3 HCOOCH=C(CH3)2 HCOOC(CH3)CH=CH2 HCOOCHC(CH3)=CH2 HCOOCH(CH2CH3)=CH2Θ§Υυ“‘ΖϊΚœΧθΦΰΒΡ”–8÷÷ΘΜΤδ÷–ΚΥ¥≈Ι≤’ώ«βΤΉΈΣ3ΉιΖεΘ§«“ΖεΟφΜΐ÷°±»ΈΣ6ΘΚ1ΘΚ1ΒΡ «![]() ΓΘ
ΓΘ
Ι ¥πΑΗΈΣΘΚ8ΘΜ![]() ΓΘ
ΓΘ
6. C H2=CH2ΚΆHBrΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…CH3CH2BrΘ§CH3CH2BrΚΆNaCNΖΔ…ζ»Γ¥ζΖ¥”Π…ζ≥…CH3CH2CNΘ§CH3CH2CN‘ΎΦν–‘ΧθΦΰœ¬ΖΔ…ζΥ°ΫβΖ¥”Π»ΜΚσΥαΜ·ΒΟΒΫCH3CH2COOHΘ§Υυ“‘ΤδΚœ≥…¬ΖœΏΈΣ
C H2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOHȧ
CH3CH2COOHȧ
Ι ¥πΑΗΈΣΘΚC H2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOHΓΘ
CH3CH2COOHΓΘ


 ΩΎΥψ–ΓΉ¥‘ΣΩΎΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗ
ΩΎΥψ–ΓΉ¥‘ΣΩΎΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗ ΧλΧλΝΖΩΎΥψœΒΝ–¥πΑΗ
ΧλΧλΝΖΩΎΥψœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΗΏΨέΈο![]() Θ®ΫαΙΙΦρ Ϋ»γΆΦΥυ ΨΘ© «“Μ÷÷ά¥‘¥”Ύ…ζΈο―ßΝιΗ–ΒΡ–¬–Ά’≥ΚœΦΝΘ§Τδ‘≠Νœ»Γ≤Ρ”Ύ÷≤ΈοΚΆξί±¥ΓΘœ¬Ν–ΙΊ”ΎΗΏΨέΈο
Θ®ΫαΙΙΦρ Ϋ»γΆΦΥυ ΨΘ© «“Μ÷÷ά¥‘¥”Ύ…ζΈο―ßΝιΗ–ΒΡ–¬–Ά’≥ΚœΦΝΘ§Τδ‘≠Νœ»Γ≤Ρ”Ύ÷≤ΈοΚΆξί±¥ΓΘœ¬Ν–ΙΊ”ΎΗΏΨέΈο![]() ΒΡΥΒΖ®¥μΈσΒΡ «Θ® Θ©
ΒΡΥΒΖ®¥μΈσΒΡ «Θ® Θ©
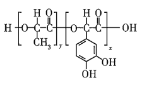
A.ΗΏΨέΈο![]() ΒΡΒΞΧε÷°“ΜΈΣ
ΒΡΒΞΧε÷°“ΜΈΣ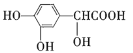
B.ΗΏΨέΈο![]() ‘Ύ“ΜΕ®ΧθΦΰœ¬ΡήΖΔ…ζΥ°ΫβΖ¥”Π
‘Ύ“ΜΕ®ΧθΦΰœ¬ΡήΖΔ…ζΥ°ΫβΖ¥”Π
C.ΖΔ…ζΨέΚœΖ¥”Π ±Θ§…ζ≥…![]() ΒΡΆ§ ±Θ§Μα”–
ΒΡΆ§ ±Θ§Μα”–![]() …ζ≥…
…ζ≥…
D.![]() ΉνΕύΩ…”κ
ΉνΕύΩ…”κ![]() ΖΔ…ζΦ”≥…Ζ¥”Π
ΖΔ…ζΦ”≥…Ζ¥”Π
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ»Φ…’0.1 molΡ≥”–ΜζΈοΒΟ0.2 mol CO2ΚΆ0.3 mol H2OΘ§”…¥ΥΒΟ≥ωΒΡΫα¬έ≤Μ’ΐ»ΖΒΡ «(ΓΓΓΓ)
A. ΗΟ”–ΜζΈοΖ÷Ή”ΒΡΫαΙΙΦρ ΫΈΣCH3ΓΣCH3
B. ΗΟ”–ΜζΈο÷–ΧΦΓΔ«β‘ΣΥΊ‘≠Ή” ΐΡΩ÷°±»ΈΣ1ΓΟ3
C. ΗΟ”–ΜζΈοΖ÷Ή”÷–≤ΜΩ…ΡήΚ§”–![]() ΥΪΦϋ
ΥΪΦϋ
D. ΗΟ”–ΜζΈοΖ÷Ή”÷–Ω…ΡήΚ§”–―θ‘≠Ή”
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ‘ΎCF3COOHΥ°»ή“Κ÷–ΫΪΦΉΆι÷±Ϋ”ΉΣΜ·ΈΣCF3COOCH3Θ®Υ°Ϋβ…ζ≥…CH3OHΘ©ΒΡΖ¥”ΠΜζάμ»γΆΦΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
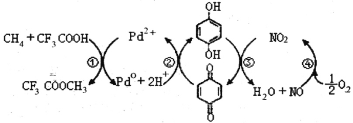
A.ΥυΚ§≤ΩΖ÷‘ΣΥΊΒΎ“ΜΒγάκΡήΘΚC<N<O<F
B.Ζ¥”ΠΔέΒΡ―θΜ·ΦΝΈΣΕ‘±ΫΕΰΖ”
C.…œ ωΖ¥”ΠΒΡΉήΖ¥”Π ΫΈΣΘΚCH4+CF3COOH+![]() O2
O2 CF3COOCH3+H2O
CF3COOCH3+H2O
D.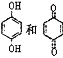 ΨυΩ…“‘”κ≈®δεΥ°Ζ¥”ΠΘ§«“Ζ¥”Πάύ–ΆœύΆ§
ΨυΩ…“‘”κ≈®δεΥ°Ζ¥”ΠΘ§«“Ζ¥”Πάύ–ΆœύΆ§
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ»βΙπΝρΑΖΘ®CinanserinΘ© «…œ άΦΆ70Ρξ¥ζ”Ο”ΎΩΙΨΪ…ώΖ÷Ν―÷ΔΒΡ“©ΈοΘ§Ε‘ΙΎΉ¥≤ΓΕΨ3CLΥ°ΫβΟΗΨΏ”–“÷÷ΤΉς”ΟΘ§œ¬ΆΦ «ΤδΚœ≥…¬ΖœΏΆΦΓΘ

“―÷Σ“‘œ¬–≈œΔΘΚ
ΔΌAΈΣ≥ΘΦϊΒΡΧΰΘ§ΥϋΕ‘H2ΒΡœύΕ‘ΟήΕ»ΈΣ39
ΔΎ2RCOOH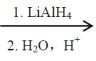 RCH2OH
RCH2OH
Δέ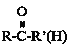 +HCN
+HCN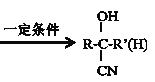
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©AΒΡΟϊ≥Τ__________ΘΜBΒΡΫαΙΙΦρ Ϋ___________ΘΜ»βΙπΝρΑΖ÷–Κ§―θΙΌΡήΆ≈ΒΡΟϊ≥Τ__________ΓΘ
Θ®2Θ©≤Ϋ÷ηΔΌΒΡΖ¥”ΠΖΫ≥Χ Ϋ________________Θ§ΤδΖ¥”Πάύ–ΆΈΣ_________ΓΘ
Θ®3Θ© ÷–Ι≤ΤΫΟφΒΡ‘≠Ή”ΉνΕύ________ΗωΓΘ
÷–Ι≤ΤΫΟφΒΡ‘≠Ή”ΉνΕύ________ΗωΓΘ
Θ®4Θ©M «CΒΡΆ§Ζ÷“λΙΙΧεΘ§ΖϊΚœœ¬Ν–ΧθΦΰΒΡM”–_______÷÷ΓΘ
ΔΌ τ”ΎΖΦœψΉεΜ·ΚœΈοΓΘΔΎΡήΖΔ…ζ“χΨΒΖ¥”ΠΓΘ
Θ®5Θ©≤Έ’’ΧβΗ…Θ§–¥≥ω”…![]() ÷Τ±Η
÷Τ±Η![]() ΒΡΝς≥ΧΆΦΓΘ_______ΓΘ
ΒΡΝς≥ΧΆΦΓΘ_______ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ“―÷ΣΘΚCH3CH2CH2CH2OH![]() CH3CH2CH2CHOΘΜάϊ”Ο»γΆΦΉΑ÷ϔϒΐΕΓ¥ΦΚœ≥…’ΐΕΓ»©œύΙΊ ΐΨί»γ±μΘΚ
CH3CH2CH2CHOΘΜάϊ”Ο»γΆΦΉΑ÷ϔϒΐΕΓ¥ΦΚœ≥…’ΐΕΓ»©œύΙΊ ΐΨί»γ±μΘΚ
Έο÷ | Ζ–Βψ/Γφ | ΟήΕ» / gcm-3 | Υ°÷–»ήΫβ–‘ |
|
’ΐΕΓ¥Φ | 117.2 | 0.8109 | ΈΔ»ή | |
’ΐΕΓ»© | 75.7 | 0.8017 | ΈΔ»ή |
œ¬Ν–ΥΒΖ®÷–Θ§≤Μ’ΐ»ΖΒΡ «
A.ΈΣΖά÷Ι≤ζΈοΫχ“Μ≤Ϋ―θΜ·Θ§”ΠΫΪΥαΜ·ΒΡNa2Cr2O7»ή“Κ÷πΒΈΦ”»κ’ΐΕΓ¥Φ÷–
B.Β±Έ¬Ε»ΦΤ1 Ψ ΐΈΣ90~95ΓφΘ§Έ¬Ε»ΦΤ2 Ψ ΐ‘Ύ76ΓφΉσ”“ ±Θ§ ’Φ·≤ζΈο
C.Ζ¥”ΠΫα χΘ§ΫΪΝσ≥ωΈοΒΙ»κΖ÷“Κ¬©ΕΖ÷–Θ§Ζ÷»ΞΥ°≤ψΘ§¥÷’ΐΕΓ»©¥”Ζ÷“Κ¬©ΕΖ…œΩΎΒΙ≥ω
D.œρΜώΒΟΒΡ¥÷’ΐΕΓ»©÷–Φ”»κ…ΌΝΩΫπ τΡΤΘ§Φλ―ιΤδ÷– «ΖώΚ§”–’ΐΕΓ¥Φ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΝρ¥ζΝρΥαΡΤ(Na2S2O3) «“Μ÷÷ΫβΕΨ“©Θ§”Ο”ΎΖζΜ·ΈοΓΔ…ιΓΔΙ·ΓΔ«ΠΓΔΈΐΓΔΒβΒ»÷–ΕΨΘ§ΝΌ¥≤≥Θ”Ο”Ύ÷ΈΝΤίΓ¬ι’νΘ§ΤΛΖτπΰ―ςΒ»≤Γ÷Δ.Νρ¥ζΝρΥαΡΤ‘Ύ÷––‘ΜρΦν–‘ΜΖΨ≥÷–Έ»Ε®Θ§‘ΎΥα–‘»ή“Κ÷–Ζ÷Ϋβ≤ζ…ζSΚΆSO2
Β―ιIΘΚNa2S2O3ΒΡ÷Τ±ΗΓΘΙΛ“Β…œΩ…”ΟΖ¥”ΠΘΚ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2÷ΤΒΟΘ§ Β―ι “ΡΘΡβΗΟΙΛ“ΒΙΐ≥ΧΒΡΉΑ÷Ο»γΆΦΥυ ΨΘΚ

(1)“«ΤςaΒΡΟϊ≥Τ «_______Θ§“«ΤςbΒΡΟϊ≥Τ «_______ΓΘb÷–άϊ”Ο÷ ΝΩΖ÷ ΐΈΣ70%80%ΒΡH2SO4»ή“Κ”κNa2SO3ΙΧΧεΖ¥”Π÷Τ±ΗSO2Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_______ΓΘc÷– ‘ΦΝΈΣ_______
(2) Β―ι÷–“ΣΩΊ÷ΤSO2ΒΡ…ζ≥…ΥΌ¬ Θ§Ω…“‘≤…»ΓΒΡ¥κ ©”–_______ (–¥≥ω“ΜΧθ)
(3)ΈΣΝΥ±Θ÷ΛΝρ¥ζΝρΥαΡΤΒΡ≤ζΝΩΘ§ Β―ι÷–Ά®»κΒΡSO2≤ΜΡήΙΐΝΩΘ§‘≠“ρ «_______
Β―ιΔρΘΚΧΫΨΩNa2S2O3”κΫπ τ―τάκΉ”ΒΡ―θΜ·ΜΙ‘≠Ζ¥”ΠΓΘ
Ή ΝœΘΚFe3++3S2O32-Fe(S2O3)33-(ΉœΚΎ…Ϊ)
ΉΑ÷Ο | ‘ΦΝX | Β―ιœ÷œσ |
| Fe2(SO4)3»ή“Κ | ΜλΚœΚσ»ή“Κœ»±δ≥…ΉœΚΎ…ΪΘ§30sΚσΦΗΚθ±δΈΣΈό…Ϊ |
(4)ΗυΨί…œ ω Β―ιœ÷œσΘ§≥θ≤Ϋ≈–ΕœΉν÷’Fe3+±ΜS2O32-ΜΙ‘≠ΈΣFe2+Θ§Ά®Ιΐ_______(Χν≤ΌΉςΓΔ ‘ΦΝΚΆœ÷œσ)Θ§Ϋχ“Μ≤Ϋ÷Λ Β…ζ≥…ΝΥFe2+ΓΘ¥”Μ·―ßΖ¥”ΠΥΌ¬ ΚΆΤΫΚβΒΡΫ«Ε»Ϋβ Ά Β―ιΔρΒΡœ÷œσΘΚ_______
Β―ιΔσΘΚ±ξΕ®Na2S2O3»ή“ΚΒΡ≈®Ε»
(5)≥Τ»Γ“ΜΕ®÷ ΝΩΒΡ≤ζΤΖ≈δ÷Τ≥…Νρ¥ζΝρΥαΡΤ»ή“ΚΘ§≤Δ”ΟΦδΫ”ΒβΝΩΖ®±ξΕ®ΗΟ»ή“ΚΒΡ≈®Ε»ΘΚ”ΟΖ÷ΈωΧλΤΫΉΦ»Ζ≥Τ»ΓΜυΉΦΈο÷ K2Cr2O7(ΡΠΕϊ÷ ΝΩΈΣ294gmol-1)0.5880gΓΘΤΫΨυΖ÷≥…3ΖίΘ§Ζ÷±πΖ≈»κ3ΗωΉΕ–ΈΤΩ÷–Θ§Φ”Υ°≈δ≥…»ή“ΚΘ§≤ΔΦ”»κΙΐΝΩΒΡKI≤ΔΥαΜ·Θ§ΖΔ…ζœ¬Ν–Ζ¥”ΠΘΚ6I-+Cr2O72-+14H+ = 3I2+2Cr3++7H2OΘ§‘ΌΦ”»κΦΗΒΈΒμΖέ»ή“ΚΘ§ΝΔΦ¥”ΟΥυ≈δNa2S2O3»ή“ΚΒΈΕ®Θ§ΖΔ…ζΖ¥”ΠI2+2S2O32- = 2I- + S4O62-Θ§»ΐ¥ΈœϊΚΡ Na2S2O3»ή“ΚΒΡΤΫΨυΧεΜΐΈΣ25.00 mLΘ§‘ρΥυ±ξΕ®ΒΡΝρ¥ζΝρΥαΡΤ»ή“ΚΒΡ≈®Ε»ΈΣ_______molL-1
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΘ®1Θ©Θ®CH3Θ©3 NH+ΚΆ![]() Ω…–Έ≥…άκΉ”“ΚΧεΓΘάκΉ”“ΚΧε”…“θΓΔ―τάκΉ”Ήι≥…Θ§»έΒψΒΆ”Ύ100ΓφΘ§ΤδΜ”ΖΔ–‘“ΜΑψ±»”–Μζ»ήΦΝ___________Θ®ΧνΓΑ¥σΓ±ΜρΓΑ–ΓΓ±Θ©Θ§Ω…”ΟΉς___________Θ®Χν–ρΚ≈Θ©ΓΘ
Ω…–Έ≥…άκΉ”“ΚΧεΓΘάκΉ”“ΚΧε”…“θΓΔ―τάκΉ”Ήι≥…Θ§»έΒψΒΆ”Ύ100ΓφΘ§ΤδΜ”ΖΔ–‘“ΜΑψ±»”–Μζ»ήΦΝ___________Θ®ΧνΓΑ¥σΓ±ΜρΓΑ–ΓΓ±Θ©Θ§Ω…”ΟΉς___________Θ®Χν–ρΚ≈Θ©ΓΘ
a.÷ζ»ΦΦΝ b.ΓΑ¬Χ…ΪΓ±»ήΦΝ c.Η¥Κœ≤ΡΝœ d.Ψχ»»≤ΡΝœ
Θ®2Θ©ΈΣΝΥ―–ΨΩ‘ΎΡ…ΟΉΦΕΒΡΩ’Φδ÷–Υ°ΒΡΫα±υΈ¬Ε»Θ§ΩΤ―ßΦ“Ε‘≤ΜΆ§÷±ΨΕΧΦΡ…ΟΉΙή÷–Υ°ΒΡΫα±υΈ¬Ε»Ϋχ––Ζ÷ΈωΓΘ»γΆΦ «ΥΡ÷÷≤ΜΆ§÷±ΨΕΧΦΡ…ΟΉΙή÷–ΒΡ±υ÷υΫαΙΙΦΑΫα±υΈ¬Ε»Θ§±υ÷υΒΡ¥σ–Γ»ΓΨω”ΎΧΦΡ…ΟΉΙήΒΡ÷±ΨΕΓΘΥ°‘ΎΧΦΡ…ΟΉΙή÷–Ϋα±υΒΡΙφ¬… «_________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΘ®1Θ©Ρ≥Έο÷ ÷ΜΚ§CΓΔHΓΔO»ΐ÷÷‘ΣΥΊΘ§ΤδΖ÷Ή”ΡΘ–Ά»γΆΦΥυ ΨΘ§Ζ÷Ή”÷–Ι≤”–12Ηω‘≠Ή”ΓΘ
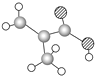
ΗΟΈο÷ ΒΡΫαΙΙΦρ ΫΈΣ_______________Θ°ΗΟΈο÷ ÷–ΥυΚ§ΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣ______________________Θ°
Θ®2Θ©œ¬Ν–ΗςΉιΈο÷ ΘΚ ΔΌ O2ΚΆO3 ΔΎ H2ΓΔD2ΓΔT2 Δέ 12CΚΆ14C Δή CH3CH2CH2CH3 ΚΆ (CH3)2CH2CH3 Δί““ΆιΚΆΕΓΆι ΔόCH3CH2CH2CHΘ®C2H5Θ©CH3ΚΆCH3CH2CH2CHΘ®CH3Θ©C2H5 ΜΞΈΣΆ§œΒΈοΒΡ «__________Θ§ ΜΞΈΣΆ§Ζ÷“λΙΙΧεΒΡ «________Θ§ΜΞΈΣΆ§ΈΜΥΊΒΡ «________Θ§ ΜΞΈΣΆ§ΥΊ“λ–ΈΧεΒΡ «_________Θ§ «Ά§“ΜΈο÷ ΒΡ «__________ΓΘ
Θ®3Θ©Ζ”ΧΣ «≥Θ”ΟΒΡΥαΦν÷Η ΨΦΝΘ§ΤδΫαΙΙΦρ Ϋ»γΆΦΥυ ΨΘΚ
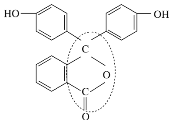
ΔΌΖ”ΧΣΒΡΖ÷Ή” ΫΈΣ___________________________________ΓΘ
ΔΎ¥”ΫαΙΙ…œΖ÷ΈωΖ”ΧΣΩ…Ω¥Ής_____________________ΓΘ
AΘ°œ©ΧΰΓΓΓΓΓΓΓΓΓΓΓΓ BΘ°ΖΦœψΜ·ΚœΈο
CΘ°¥ΦάύΈο÷ DΘ°Ζ”άύΈο÷
EΘ°Ο―άύΈο÷ FΘ°θΞάύΈο÷
ΔέΖ”ΧΣΫαΙΙΦρ Ϋ÷–Μ≠–ιœΏΒΡΒΊΖΫ «Ο―Φϋ¬πΘΩ_________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com