



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
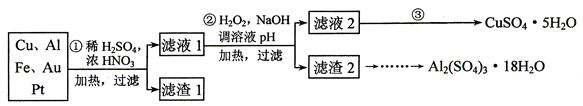
�鿴�𰸺ͽ���>>
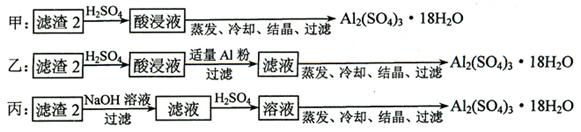
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ۢܢ� | B���٢ڢܢ� | C���ۢڢܢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
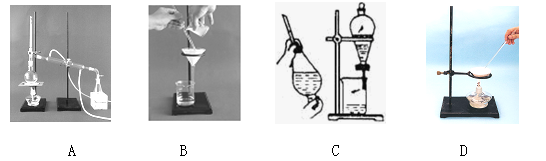
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
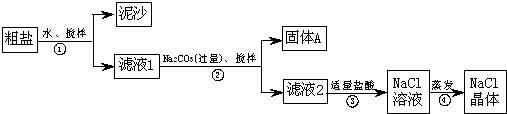
| ���� | ��֤�ķ��� | ���� | ���� |
| �������A�к� CaCO3��MgCO3 | ȡ��������A���Թ��У��μ�ϡ���ᣬ����Ϳ�г���ʯ��ˮ��С�ձ������Թܿ� | ____________ | �������� |
| �������A�к� BaCO3 | ȡ��������A���Թ��У��ȵ���________���ٵ���Na2SO4��Һ | �����ݷų����ް�ɫ���� | ___________ |
| ���������Ƶõ�NaCl�����л�����Na2SO4 | ȡ����NaCl���������Թ��е�����ˮ��________ | ____________ | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ | B��ʯ����Һ | C��KOH��Һ | D��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ֧�ܿ� |
| B����Һʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| C��ŨH2SO4ϡ��ʱ��Ӧ��ŨH2SO4�����ӵ�H2O�У�����ʱ�������ȴ |
| D���þƾ���ȡ��ˮ�еĵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��һ������HBr�����ܺ���CO2��Cl2 | B��һ������CO2 |
| C��һ������NH3��Cl2 | D�����ܺ���Cl2��CO2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com