
�ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ���£�
������ͼ�����������գ�
��1�����Դ���������ĵ缫��������ҺpHֵ��ѡ����䡢�����½��������Դ���������ĵ缫�� ��������ü��ϲ���ķ����� ��
��2��д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ ��
��3�����������SO �����ϸߣ��������ӱ�ʽ����ȥSO
�����ϸߣ��������ӱ�ʽ����ȥSO ���ñ��Լ���������ѡ��A.B.c����ѡ�۷֣�
���ñ��Լ���������ѡ��A.B.c����ѡ�۷֣�
A��Ba(OH)2 B��Ba(NO3)2 C��BaCl2
��4���жϱ��Լ��Ѿ������ķ����� ��
��5��Ϊ��Ч��ȥCa2+��Mg2+��SO �������Լ��ĺ���˳��Ϊ��ѡ��a��b��c��ѡ�۷֣�
�������Լ��ĺ���˳��Ϊ��ѡ��a��b��c��ѡ�۷֣�
A���ȼ�NaOH��Һ�����Na2CO3��Һ���ټӱ��Լ�
B���ȼ�NaOH��Һ����ӱ��Լ����ټ�Na2CO3��Һ
C���ȼӱ��Լ������NaOH��Һ���ټ�Na2CO3��Һ
��6��Ϊ���龫�δ��ȣ�������150 mL0.2 mol/LNaCl�����Σ���Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ����� ��
��1������ ���� �����۵⻯����ֽ��ˮ��ʪ��ճ�ڲ�����һ��,����װ�д�������ļ���ƿ �������ɫ������ʹ��ֽ����ɫ,֤������Cl2��
��2����ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H2O  2NaOH+Cl2��+H2��
2NaOH+Cl2��+H2��
��3��C
��4����ֹ�����ϲ���Һ�м����μ�BaCl2���粻�����������ѹ�����
��5��B.C��
��6��δ�ò��������� 100 mL������ƿ��������150 mL ��Һ
���������������1�����Դ��������Ϊ����������������NaOH�����ɼ����pH���ߣ�����������Ϊ���������������������۵⻯����ֽ��ˮ��ʪ��ճ�ڲ�����һ��,����װ�д�������ļ���ƿ �������ɫ������ʹ��ֽ����ɫ,֤������Cl2��
��2����ⱥ��ʳ��ˮ�ķ�Ӧ��2NaCl+2H2O  2NaOH+Cl2��+H2��
2NaOH+Cl2��+H2��
��3�����ӱ��Լ���ȥSO42-��ע�ⲻ�������µ����ʣ�ѡBa��NO3��2������������������ӣ����Ըñ��Լ�����ѡ�á�ͬ��Ba(OH)2��������OH-���ӡ�
��4����ֹ�����ϲ���Һ�м����μ�BaCl2���粻�����������ѹ�����
SO42-��Ca2+��Mg2+�ȷֱ���BaCl2��Һ��Na2CO3��Һ��NaOH��Һ��Ӧ���ɳ���������ͨ�����˳�ȥ��Na2CO3��Һ�ܳ�ȥ������BaCl2��Һ�������ܳ�ȥ������Na2CO3��Һ��NaOH��Һ������Ӧ�ȼ�BaCl2��Һ�ټ�Na2CO3��Һ�����������ᡣ�ʴ�Ϊ:B.C��
��6��δ�ò�����������100 mL������ƿ��������150 mL ��Һ��
���㣺���ء�ʵ���ҳ����ʡ����ӷ���ʽ��д��


 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̫���ܵ�ؿ��������ĵ�Դ(��ͼ)��
(1)��c��d��Ϊ���Ե缫���������ҺΪ����ͭ��Һ���������У�c��������������������������壬��c��Ϊ________�����ڵ������У���Һ��pH________(���������С�����䡱)��ֹͣ����Ϊʹ��Һ�ָ���ԭ��ҺӦ����������________��
(2)��c��d��Ϊͭ�缫���������ҺΪ�Ȼ�����Һ������ʱ����Һ�������ӵ����ʵ�����________(���������С�����䡱)��
(3)����ʯī�������缫���ϣ�����װ��һ��������ˮ����װ�á���ԭ���ǣ������Լ�������ˮ��pH��5.0��6.0֮�䡣��ͨ��Դ���������������彫�������ˮ���γɸ�������ȥ����ѡ�������ã�������������ɫ�������������ԣ���������������������۾������á���װ���У������ĵ缫��ӦʽΪ_______________________________________��
���������ɵ���ɫ������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��̼���̿����Ҫ�ɷ���MnCO3��MnO2��FeCO3��MgO��SiO2��Al2O3�ȡ���֪̼����������ˮ��һ������������Ĥ��ⷨ���¼��������ڴ�̼���̿�����ȡ�����̣��������£�
������Ĥ�����װ����ͼ��ʾ��
��1��д����ϡ�����ܽ�̼���̷�Ӧ�����ӷ���ʽ ��
��2���ڽ���Һ����Ԫ��ֻ��Mn2+����ʽ���ڣ���������Ҳ��MnO2�������ԭ�� .
��3����5�֣���֪��ͬ����������������������������pH���±���
�Ӱ�ˮ������Һ��pH����6���������ijɷ��� ����Һ�к��е���������H+�� ��
��4�����װ���м�ͷ��ʾ��Һ���������ƶ��ķ�����A�缫�� ����ʵ�������У�������ϡ����Ϊ���Һ�������ĵ缫��ӦʽΪ ��
��5���ù���֮���Բ��������ӽ���Ĥ����Ϊ�˷�ֹMn2+������������������Ӧ����MnO2������˷ѣ�д���ø���Ӧ�ĵ缫��Ӧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾװ�ý���ʵ�飬���ش��������⣺

��1���ж�װ�õ����ƣ�A��Ϊ__________________��B��Ϊ________________________��
��2������Ϊ_________�����缫��ӦʽΪ________________________��ͭ��Ϊ________�����缫��ӦʽΪ________________________ ��ʯī��C1Ϊ_______�����缫��ӦʽΪ____________________________��ʯī��C2����������ʵ������Ϊ________����Ӧ������B����Һ��pHֵ________��(����С�����䣬������������ˮ)
��3����C2������224 mL���壨��״���£�����������________�����ӻ���٣�__________g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪ���װ�ã�X��YΪ�缫���ϣ�aΪ�������Һ��
��1����aΪ���з�̪��KCl��Һ��XΪFe��YΪʯī�����һ��ʱ���:
X�缫�����ɹ۲쵽��ʵ�������� ��
д��Y�缫�ĵ缫��Ӧʽ ��
��2����Ҫʵ��Cu +H2SO4��CuSO4+H2����
��Y�缫������ ��
д��X�缫�ĵ缫��Ӧʽ ��
��3����Ҫ���ø�װ��������Ʒ�������һ��������aΪ ����Ӧǰ���缫��������ȣ���Ӧ��缫�������2.16g����ù���������ͨ���������ĵ�����Ϊ ��
��4����X��Y��Ϊ���Ե缫��aΪNaOH��Һ�����һ��ʱ�����Һ��pH ����������䡱����С��������Ҫʹ��Һ�ָ�ԭ����״̬��������Һ�м��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о���ѧϰС����̽��ԭ��ص��γ�����������ͼ��ʾװ�ý���ʵ��
| ��� | A | B | �ձ��е�Һ�� | ָ���Ƿ�ƫת |
| 1 | Zn | Cu | ϡ���� | �� |
| 2 | Zn | Zn | ϡ���� | �� |
| 3 | Cu | C | �Ȼ�����Һ | �� |
| 4 | Mg | Al | ����������Һ | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס������صĵ缫������ͼ��ʾ���밴Ҫ��ش��������⣺
(1)�������о�ΪCu(NO3)2��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е� ���������̼���������ҳ��е� ����
������������������
���ҳ��������Ϸ����ĵ缫��Ӧ����ʽ�� ��
(2)�������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�Ļ�ѧ����ʽ�� ��
�ڼ׳���̼���ϵ缫��Ӧ����ʽ�� ���ҳ�̼����
�缫��Ӧ���� ���������Ӧ����ԭ��Ӧ������
�۽�ʪ��ĵ���KI��ֽ�����ҳ�̼��������������ֽ��������һ��ʱ����ַ���
��ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2������������Ӧ��C12��I2���ʵ���
֮��Ϊ5��1��������HCl����һ��ǿ�ᣬ��ǿ��Ļ�ѧʽΪ ��
�����ҳ���ת��0��1 mol e-��ֹͣʵ�飬������Һ�����1L������Һ���Ⱥ��pH= �������������ɵ������ܽ�����Һ�е������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�Ǽ״�ȼ�ϵ�ع�����ʾ��ͼ������A��B��D��Ϊʯī�缫��CΪͭ�缫������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��
��1�����и����ĵ缫��ӦʽΪ____ ��
��2������A�������������ڱ�״���µ����Ϊ____ ��
��3����װ����Һ�н��������ӵ����ʵ�����ת�Ƶ��ӵ����ʵ����仯��ϵ��ͼ����ͼ�Т��߱�ʾ���� ���ӵı仯����Ӧ������Ҫʹ��װ���н���������ǡ����ȫ��������Ҫ mL 5��0 mol/L NaOH��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��9�֣���ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�á�
��ش��������⣺ 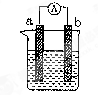
��1�����缫aΪAl���缫bΪCu���������ҺΪϡ����ʱ�������ĵ缫��ӦʽΪ�� ��
��2�����缫aΪAl���缫bΪMg���������ҺΪ����������Һʱ����װ��
����ܡ����ܡ����γ�ԭ��أ������ܣ���˵�����ɣ����ܣ���ָ�������������ϣ� ������Ӧ���ռ�����״����224mL����ʱ�����ĵĵ缫����Ϊ g��
��3��ȼ�ϵ�صĹ���ԭ���ǽ�ȼ�Ϻ�����������O2����Ӧ�������Ļ�ѧ��ֱ��ת��Ϊ���ܡ������һȼ�ϵ�أ��Ե缫aΪ�������缫bΪ����������Ϊȼ�ϣ���������������ҺΪ���Һ�������Ӧͨ�� ������a��b����ͬ�������Ӵ� ���������������Һ��OH���� ���ƶ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com