
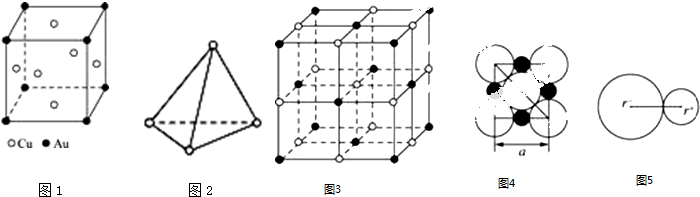
���� ��1�����þ�̯�����㻯ѧʽ��
��2��P4����Ϊ���������ͷ��ӣ�P4��������6��P-P����P4����Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�
��3�������þ�̯������ÿ�������к��е������Ӻ������Ӹ�����
�ڸ���ͼƬ��ȷ��NaCl�������������ӵ���̾��룮
��� �⣺��1��Auԭ��λ�ڶ��㣬��ĿΪ8��$\frac{1}{8}$=1��Cuλ�ھ������ģ���ĿΪ6��$\frac{1}{2}$=3����ѧʽ��дΪ��Cu3Au��
�ʴ�Ϊ��Cu3Au��
��2����P4����Ϊ���������ͷ��ӣ�ÿ����Ϊ�������Σ�P4��������6��P-P����1mol P4��������6mol P-P�����ʴ�Ϊ��6��
��P4����Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�
�ʴ�Ϊ���Ǽ��ԣ�
��3���ٸ��ݾ���ͼ����NaCl�����У�ÿ��Na+ͬʱ����6�������ӣ�ÿ���þ����������Ӹ���=12��$\frac{1}{4}$=4�������Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�����Ӹ�����Ϊ1��1��
�ʴ�Ϊ��6��1��1��
�ڸ���ͼƬ��֪��NaCl�������������ӵ���̾���Ϊa��һ�뼴$\frac{a}{2}$�������ӵİ뾶Ϊ�Խ��ߵ�$\frac{1}{4}$����Ϊ$\frac{\sqrt{2}a}{4}$�������ӵİ뾶Ϊ$\frac{a}{2}-\frac{\sqrt{2}a}{4}$������Na+���Ӱ뾶��Cl-���Ӱ뾶֮��Ϊ $\frac{{r}^{+}}{{r}^{-}}$=$\frac{\frac{a}{2}-\frac{\sqrt{2}a}{4}}{\frac{\sqrt{2}a}{4}}$=0.414��
�ʴ�Ϊ��0.414��
���� ���⿼���йؾ���ļ��㣬��������н�ǿ�ķ����Ժ����ԣ�ѧϰ��ע�⾧�����ȷ���������ѧ���жϾ�������λ���ļ��㣮


 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��� | ʱ��/min ���ʵ���/mol �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 |
| 1 | 800 | 1.0 | 0.80 | 0.67 | 0.57 | 0.50 | 0.50 |
| 2 | 800 | 1.0 | 0.60 | 0.50 | 0.50 | 0.50 | 0.50 |
| 3 | 820 | 1.0 | 0.40 | 0.25 | 0.20 | 0.20 | 0.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ϊp����ǡ�8�����εģ�����p�ĵ����ߡ�8������ | |
| B�� | K�ܼ���3s��3p��3d��3f�ĸ���� | |
| C�� | ̼ԭ�ӵ�2p����������������෴�ĵ��� | |
| D�� | ����˵��������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 �������еĻ�ѧ�������Ӽ������Թ��ۼ������������ӻ��������ӡ����ۡ�����
�������еĻ�ѧ�������Ӽ������Թ��ۼ������������ӻ��������ӡ����ۡ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| A | ||||
| E | B | C | D |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com