
(1)�����й�ʵ��������жϲ���ȷ����________(���й���ţ�ѡ�����۷�)��
A����10mL��Ͳ��ȡϡ������Һ8.0mL
B���ø����pH��ֽ�ⶨ��ˮ��pH
C���ü�ʽ�ζ�����ȡKMnO4��Һ19.60mL
D��ʹ������ƿ������Һʱ������Һ�涨�ݺ�������Һ��Ũ��ƫ��
E��Բ����ƿ����ƿ�����������ʱ��Ӧ����ʯ������
F���ⶨ����ͭ�����нᾧˮ����ʱ�������Ⱥ���������ڸ���������ȴ���ٳ���
G���ñ����ᣬ����ˮ������ƿ����������pHΪ1�Ĵ���ϡ��Һ
(2)ʵ������������ͼװ�ý����к��ȵIJⶨ��

�ش��������⣺
�ٸ�ͼ��������δ������������________��________��
�������0.5mol/L��������������ƹ������ʵ�飬��ʵ����������ġ��к��ȡ���(�ƫ����ƫС�����䡱)��ԭ����________��
(3)�ڷ�����ѧ�г���Na2C2O4������Ϊ�����ʲⶨKMnO4��Һ��Ũ�ȣ���H2SO4��Һ�У���Ӧ���£�
2MnO4����5C2O42����16H+![]() 2Mn2+��10CO2����8H2O
2Mn2+��10CO2����8H2O
����������ƽ��ȡWgNa2C2O4���壮
�ڽ�WgNa2C2O4���100mL����Һ����ȡ20.00mL������ƿ�У�������KMnO4��ҺӦװ��________(���ʽ����ʽ��)�ζ����У�
�����ζ��ܵ���ʼ�������յ������ͼ��ʾ��������KMnO4�����ʵ���Ũ��Ϊ________(�����ʽ)��
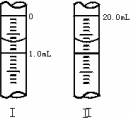
�����ζ���������������ⶨ��KMnO4��Һ��Ũ��________(�ƫ�ߡ���ƫ�͡����䡱)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʾΪ���������IJ��ֽṹ��
����ͼ��ʾΪ���������IJ��ֽṹ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����������Һ ����ʽ��NaOH ��Է���������40 �ܶȣ�1.2g?cm-3 ����������20%��1����NaOH��Һ�����ʵ���Ũ��Ϊ 6 6 mol/L����2������Ҫ���Ƹ�Ũ�ȵ�NaOH��Һ100ml������� 24.0 24.0 g�����������ƣ���Һ���Ƶ�����Ļ����������£� ��3��������ʵ�鲽��A��F��ʵ������Ⱥ�������� CBDFAE CBDFAE ����4������ʵ�鲽��A��B��E��F���õ�����������Ϊ 100ml����ƿ 100ml����ƿ ����5�����в�����NaOH��Һ�����ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ ƫ�� ƫ�� ��������ƿ��ԭ����������ˮ ��Ӱ�� ��Ӱ�� ���۶���ʱ���ӹ۲�Һ�� ƫ�� ƫ�� ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ��Ϥ��ѧʵ����������;���÷�������ȷ����ʵ����������û�ѧʵ���ǰ�ᣮ���������һЩʵ���װ�û����ͼ���밴Ҫ��ش��й����⣮ ��1����ͼ1��ʾ��ijЩ�������ȡװ�ã��������ô�װ����ȡ���岢�ܡ��濪���á������ͣ������ B B ������ţ���ͬ���� A���ô���ʯ��ϡ������ȡ������̼ B����п����ϡ���������� C����Ũ���������������ȡ�������� D���ø�����ع����Ũ������ȡ���� ��2��ʵ���ҿ�����ͼ2��ʾװ�ø��ﲢ�ռ�ij����R����R������ B B ��A��SO2 B��NH3 C��NO D��Cl2 ��3��ijͬѧ����ͼ3��ʾװ�ý���ʵ�飬ʵ������г���С�����������������ʹ�õĹ����Һ���Լ������� C C ��A��ͭ��Ũ���� B������Ũ���� C����������������Һ D���������̺�Ũ���� ��4��ָ��ͼ4����ʵ���и����ڵ�һ������ �� ��ͷ�ι������Թ��ڲ� ��ͷ�ι������Թ��ڲ� ���� �Թܿ�������б �Թܿ�������б ���鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ������ʡ�������2010��2011ѧ���һ��ѧ�����п��Ի�ѧ�������� ���ͣ�058 �йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ��(�г�װ��һ����ʡ��)����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���b��c�����н���(��Ъ��)ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺
(1)C����ˮ�����ã�________�� (2)M�������ķ�Ӧ�Ļ�ѧ����ʽΪ��________�� (3)��M�пɹ۲쵽������________���п���ʶ����ʵ������д���________(��μӡ����μӡ�)��ѧ��Ӧ����������ʶ���÷�Ӧ�Ĵ�������Ҫһ����________�� (4)ʵ��һ��ʱ�����������ƾ��ƣ���Ӧ���ܼ������У���ԭ����________�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ���㽭ʡģ���� ���ͣ��ƶ��� ������������ר�Ҷ�ʯ�ҵ����½����������о�����Ҳʹ��ʯ�ҵ���һ���ϵķ����ֻ������µĻ�����ʯ�ҵ�����Ca��N��C������Ԫ����ɵ��Σ��京�ơ��������������ֱ�Ϊ50%��35%��ʯ�ҵ���ȫˮ���IJ����ǹ���A������B������B����ȡ���ʵ���Ҫԭ�ϡ�����A�ڸ����·ֽ�����D������C��B�Ĵ���������ΪE��F��F����������IJ���G����E����һ�ֹ�ҵǿ�ᡣ��G��F�Ļ�����ܽ��ڽӽ���ȵ�ˮ�У��������������ˮ��Һ���÷�Ӧ�ɱ�ʾ����G+F+H2O��2HNO2������ƽ���� ��ش��������⣺ ��1��ʯ�ҵ��Ļ�ѧʽΪ__________________�� ��2��д������B��C���Ȼ�����Һ��Ӧ�Ļ�ѧ����ʽ��__________________�� ��3�������ᣨHNO2����һ������������൱�����ᣬ�ܲ��ȶ���ͨ���������������ֽ⡣�����������£���NaNO2��KI�����ʵ���1:1ǡ����ȫ��Ӧ����I-������ΪI2ʱ����������Ϊ__________���ѧʽ����Ҫ�õ��ȶ�HNO2��Һ���������䶳��ŨNaNO2��Һ�м����ͨ��ij�����ʣ��������ʲ��ʺ�ʹ����_________ ������ţ��� a. ���� b. ������̼ c. ϡ���� d. �������� e. ������ ��4����ҵ��ˮ�е�NO2-�������۳�ȥ����֪�˷�Ӧ��ϵ�а���Al��NaAlO2��NaNO2��NaOH��NH3��H2O�������ʡ�д��������Ӧ�����ӷ���ʽ��_______________�������õ�ⷨ����ˮ��NO2- ת��ΪN2��ȥ��N2����_________����缫���ƣ����ɡ� ��5��ij�о�С��ѧ�����������ͷ��KClO3ʵ�鷽������  �йص����ӷ�Ӧ����ʽΪ_______________�� ��6���ڣ�5�����������ϣ�Ҫȷ�����ͷ�к�KClO3��������е�ʵ�����Ϊ_________�� �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |