
��7�֣�ʵ��������480 mL 0.1mol��L-1 NaOH��Һ���ش���������
(1)Ӧ��������ƽ��ȡ�������ƹ��� �� g��
(2)����NaOH��Һʱ���õ���Ҫ������������ƽ��ҩ�ס��ձ�������������Ͳ�� �� ��
(3)��ʵ���������������������Һ�����ʵ���Ũ���� A��ƫ�� B��ƫ�� C������(�÷��Żش�)
�� ����ʱ���ӿ̶��� �� ��
�� ������ֽ�ϳ���NaOH���� �� ��
��4������������Լ�ƿ��ʢ���������ƺõ���Һ���ϱ�ǩ����������ȥ(��ǩ��ͼ)��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ��������480 mL 0.1 mol��L-1Na2CO3��Һ������������⣺
��1��Ӧ��������ƽ��ȡ��ˮ̼���Ʒ�ĩ__________g��
��2�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ���ƽƽ��ʱ����ʵ�ʳ�����̼���Ʒ�ĩ��__________g��1 g���������룩��
��3������Na2CO3��Һʱ���õ���Ҫ������__________��__________��__________��__________��__________��
��4��ʹ������ƿ������Һʱ�����������ʹ������ҺŨ��ƫ�͵���__________��
������ƽ��ʹ�����룩����ʱ�����������������λ�÷ŵߵ��� ����ˮ̼���Ʒ�ĩ�Ѳ�����ˮ��ɾ��� ����Һת�Ƶ�����ƿ���ձ���������δ������ˮϴ�� ��ת����Һǰ����ƿ������������ˮ �ݶ���ʱ����������ƿ�Ŀ̶��� ���ݺ�ҡ�ȣ�����Һ�潵�ͣ��ֲ�������ˮ�����´ﵽ�̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������ʡ����12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������˵���У�һ����ȷ����
A����ʢ������Ũ���ᡢϡ�������֧�Թ��У��ֱ�����С��ͬ��ͭƬ������֤��Ũ����������ǿ��ϡ����
B������ʽ�ζ�������з�̪��NaOH��Һ�еμӱ�HCl��Һ�����������һ��HCl��Һ��ۺ�ɫ�պ���ȥ��˵���Ѵ�ζ��յ�
C��ʵ��������480 mL 0.1 mol/L NaOH��Һ����Ҫȷ����NaOH 1.920 g
D�������ھƾ��ƻ����ϼ���ʱ�ۻ��������䣬����Ϊ�������ۻ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012��㶫ʡ��ͷ�и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��14�֣�ʵ��������480 mL 0.5mol/L��NaOH��Һ������д���пհף�
��1����ʵ��������������У���ƽ��ҩ�ס��ձ�����������________________��_______________��
��2�����Ƹ���Һ��ȡNaOH����________________g��
��3��ijͬѧ���ӹ۲�Һ�棬��ʹ������ҺŨ��__________���ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ����
ijͬѧ�ܽ��δ��ȴ�����±㽫��Һת��������ƿ��������ҺŨ�Ȼ�__________
ijͬѧ�����������ƹ���ʱ����ʱ�������������ҺŨ�Ȼ�__________
��4����ʵ������г����������Ӧ��δ�����������ˮʱ���������˿̶ȣ�Ӧ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ�����и�һ��ѧ������������ѧ�Ծ� ���ͣ������
��9�֣�ʵ��������480 mL 0.1 mol��L-1 NaOH��Һ���ش��������⡣
��1��Ӧ��������ƽ��ȡ�������� g��
��2������NaOH��Һʱ���õ���Ҫ������������ƽ��Կ�ס��ձ�������������Ͳ�� �� ��
��3����ʵ��ʱ��������������������Ƶ����ʵ���Ũ��ƫ�ߡ�ƫ�ͻ��Dz��䡣
�ٶ���ʱ���ӿ̶��� ���ڷ�����ֽ�ϳ���NaOH���� ��
��4������������Լ�ƿ��ʢ���������ƺõ���Һ���ϱ�ǩ����������ȥ����ǩ����ͼ
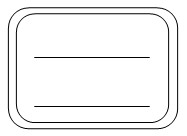
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com