
����Ŀ��ʵ���ҿ���ͭ��Ũ������Ȼ�������������Ʒ�Ӧ��ȡ��������

(1)�����������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ�ͼ�п�ѡ�õķ���װ���� ______ (��д��ĸ)��
(2)����������������Ʒ�Ӧ��ȡ3.36 L(��״��)���������������40%��������(��������)���������������ƣ����������ȡ���������� ______g(����һλС��)��
(3)ij�ȵ糧�Ͽմ��������������������س��꣬�ֶԸ�������ˮ��Ʒ����̽����������pH��ֽ�ⶨ��ˮ��Ʒ��pH����������Ϊ______�������ƷpHԼΪ3��Ϊ��һ��̽����SO2���γ���������ʣ���һ������SO2ͨ������ˮ�У����pHΪ3����Һ��Ȼ����Һ��ΪA��B���ݣ�����ҺB�����ڿ����У����ܱձ����A��ȣ����ú����ҺB��ˮ�ĵ���̶Ƚ� ______(����������������С������������)��
���𰸡�ae 31.5 ȡһ����ֽ���ڸ���ྻ�ı�����(����Ƭ)�ϣ��ø���ྻ�IJ�����պȡ��ˮ��Ʒ������ֽ���룬����Ӻ����ɫ��������ձ���ɫ�������� ��С
��������
(1)�����������������ȡSO2���Լ�Ϊ��̬��Һ̬����Ӧ����������ȣ���ͨ������������������������Ʒ�Ӧ���ʣ�
(2)�����غ�ɵã�Na2SO3��SO2�����ݹ�ϵʽ��������������ʵ����������Ҫ�������Ƶ�����������������Ƶ������������ټ������Ҫ���ʺ���������Ƶ�������
(3)�ⶨpH�����ò�����պȡ��Һ��Ȼ�����ɫ���Աȣ�����ҺB�����ڿ����У������ᱻ�����������ᣬ��Һ������ǿ��
(1)��������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ����ڷ�Ӧ����Ҫ���ȣ��ų�װ��d����������������ϸС����������ѡ��װ��c��װ��b����֪��Ӧ���ʣ��ʿ�ѡ�õķ���װ��Ϊ��ae��
(2)����������������Ʒ�Ӧ��ȡ���������ݷ�Ӧ����ʽ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O�����ݷ�Ӧ����ʽ��֪��Na2SO3��SO2��n(SO2)=![]() =0.15 mol�������������Ƶ�����Ϊ��m(Na2SO3)= 0.15 mol��126 g/mol=18.9 g���������40%��������(��������)���������������ƣ����������Ƶ���������Ϊ60%���������ȡ���������Ƶ�����Ϊ
=0.15 mol�������������Ƶ�����Ϊ��m(Na2SO3)= 0.15 mol��126 g/mol=18.9 g���������40%��������(��������)���������������ƣ����������Ƶ���������Ϊ60%���������ȡ���������Ƶ�����Ϊ![]() ==31.5 g��
==31.5 g��
(3)�ⶨpH�����ò�����պȡ��Һ������pH��ֽ�ϣ�����Ӻ����ɫ���Աȣ���������Ϊȡһ����ֽ���ڸ���ྻ�ı�����(����Ƭ)�ϣ��ø���ྻ�IJ�����պȡ��ˮ��Ʒ����pH��ֽ�ϣ�����Ӻ����ɫ���ٶ��ձ���ɫ������������ҺB�����ڿ����У������ᱻ�����������ᣬ������Һ������ǿ����Һ��c(H+)����ˮ���������������ǿ����ˮ�ĵ���̶ȼ�С��


 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪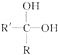 �ᷢ����Ӧ��Ϊ
�ᷢ����Ӧ��Ϊ![]() (R��R�����Ϊ������Ҳ����ΪH)����д������������NaOHˮ��Һ��ַ�Ӧ�������л���Ľṹ��ʽ��
(R��R�����Ϊ������Ҳ����ΪH)����д������������NaOHˮ��Һ��ַ�Ӧ�������л���Ľṹ��ʽ��
(1)CH3Cl��___________
(2)CH2Cl2��______________
(3)CHCl3��_________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ƻ���к��е��ۡ������Ǻ����εȣ�ij������ȤС�������һ��ʵ��֤��ijЩ�ɷݵĴ��ڣ�������벢Э������������ʵ�顣
��1����С�Թ�ȡ������ƻ��֭������_________����Һ��������ƻ���к��е��ۡ�
��2����С�Թ�ȡ������ƻ��֭����������Cu(OH)2����Һ�������ȣ�����ש��ɫ�ij�������ƻ���к���____________��д����ʽ����
��3����������һ�������¿��Եõ���ѧʽΪC2H6O�Ļ�����A��
A + CH3COOH������ζ�IJ���
�� ��A���������Ϊ75%��ˮ��Һ��������_____________��
��д��������A��CH3COOH��Ӧ�Ļ�ѧ����ʽΪ______________________���÷�Ӧ������Ϊ_____��
��4��ƻ���к���ƻ���ᣬ�������Է�������Ϊ134��ȡ0.02molƻ���ᣬʹ����ȫȼ�գ���ȼ�պ�IJ����Ⱥ�ͨ����������ˮCaCl2�ͼ�ʯ�ң����߷ֱ�����1.08g �� 3.52g���������C��Hԭ�ӵĸ�����_______��ƻ����ķ���ʽ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ҹ�����Ѹ�ͷ�չ��Ϊ���ǵij��д����˼���ı����������������·�Ӧ�������죺2Al��Fe2O3 ![]() 2Fe �� Al2O3 ����ش��������⣺
2Fe �� Al2O3 ����ش��������⣺
��1���÷�Ӧ����_______________��Ӧ��������������������������
��2���÷�Ӧ�ķ�Ӧ������_______________������ĸ����
A.���Ϸ�Ӧ B.�û���ӦC.���ֽⷴӦ D.�ֽⷴӦ
��3���÷�Ӧ�е���������_______________���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪ�������ϸ������Ҫ��Ԫ�ؼ��������������������У�������ǣ� ��

A.ͼ����ʾΪϸ����������Ҫ��Ԫ�ص���ռ����
B.��Ϊ O �ĺ�����࣬���� O �ǹ����л���������Ԫ��
C.ϸ��ʧȥ��ˮ�ֺ�C ����ռ�������
D.ͼ����ʾ��Ԫ���ڷ������Ҳ�����ҵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��SnCl4��һ�ּ���ˮ��Ļ���������Ʊ���Ҫ����ˮ�������£��ܱյ�װ���н��С����ó���(ϵͳ������ϴ�)�������ж�ʵ��ȱ��Ƚ϶࣬��ͼ������ʵ�����SnCl4 ���Ʊ�������˳��淽���ı�(��֪��SnCl4���۵�Ϊ��33�棬�е�Ϊ114.1��)��
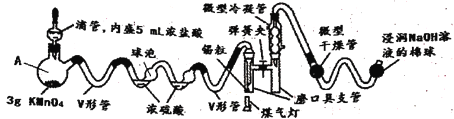
(1)���Ѹ���ĸ�����������ͼ���Ӻú���Ҫ���еIJ���Ϊ________________________��
(2)V�ιܵ�������________________________________________________________��
(3)����˵������ȷ����_____
A.����A������Ϊ������ƿ
B.Ϊ�˳�ָ���������Ũ��������Ӧ�������ݵ����
C.����ʱӦ�ȵμ�Ũ���ᣬʹ����װ���ڳ�������ɫ���壬����ú���Ƽ���
D.���ɵ�SnCl4��������ȴ�ۼ���ĥ�ھ�֧����
E.������е��Լ������Ǽ�ʯ�ҡ�������������ˮ�Ȼ��Ƶ�
(4)ʵ����0.59������ȫ��Ӧ�Ƶ�1.03g SnCl4�����ʵ��IJ���Ϊ_____________(����������һλС��)��
(5)SnCl4������ˮ�����ķ�Ӧ��������Ļ����ԭ������Ӧ�Ļ�ѧ����ʽΪ_______________��
(6)����ʵ����ŵ���________________________________________________(��д����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����³�ѹ�£�ȡ���������л����1mol���ֱ���������������ȼ�գ���������������
A. C2H5OH B. CH4 C. C2H4O D. C3H8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾװ�ý����й�ʵ�顣��ش�

(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽ��_________��
(2)װ��D���Թܿڷ��ý���NaOH��Һ��������������______��
(3)װ��B��Ӧ���õ�Һ����______��
(4)��Ӧ��ϣ�������ƿ����ͭƬʣ�࣬����ҩƷ��������֤����Ӧ���������ƿ��ȷ���������______(����ĸ)��
a.���� b.BaCl2��Һ c.���� d.NaHCO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.4molX�����0.6molY��������2L�ܱ������У�ʹ���Ƿ������·�Ӧ��4X(g)+5Y(g) ![]() nZ(g)+6W(g)��2minĩ������0.3molW������֪��Z��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.05mol/(L��min)���Լ���
nZ(g)+6W(g)��2minĩ������0.3molW������֪��Z��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.05mol/(L��min)���Լ���
��1��ǰ2min����W��Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ_______________��
��2��2minĩʱY��Ũ��Ϊ_____________________________��
��3����ѧ��Ӧ����ʽ��n=_____________________________��
��4��2minĩ���ָ�����Ӧǰ�¶ȣ���ϵ��ѹǿ�Ƿ�Ӧǰѹǿ��__________����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com