
����Ŀ���о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
��1����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2(g)+ 6H2(g) ![]() CH3OCH3(g) + 3H2O(l)���÷�Ӧ��ѧƽ�ⳣ������ʽK = ________________________��
CH3OCH3(g) + 3H2O(l)���÷�Ӧ��ѧƽ�ⳣ������ʽK = ________________________��
��2����֪��ijѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ����ƽ��ʱCO2��ת������ͼ
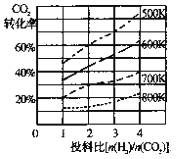
�ٸ÷�Ӧ����H ________ 0������>"����<������
�����¶Ȳ��䣬��С��ӦͶ�ϱ�[n(H2)/n(CO2)]��Kֵ��________������������������С����������������
��3��ij�¶��£������һ�����ܱ�������ͨ��CO2(g)��H2(g)����������Ӧ���������������ٷ����仯ʱ����˵����Ӧ�ﵽƽ��״̬����__________��
A.��������ɫ B.�����е�ѹǿ
C.������ܶ� D.CH3OCH3��H2O�����ʵ���֮��
��4��ij�¶��£�������ɱ���ܱ������У��ı���ʼʱ��������ʵ������ڲ�ͬ��ѹǿ�£�ƽ��ʱCH3OCH3(g)�����ʵ������±���ʾ��
P1 | P2 | P3 | |
I��2.0 mol CO2 6.0 mol H2 | 0.10 mol | 0.04 mol | 0.02 mol |
II��1.0 mol CO2 3.0 mol H2 | X1 | Y1 | Z1 |
III��1.0 mol CH3OCH3 3.0 mol H2O | X2 | Y2 | Z2 |
��P1 ________ P3������>����<������=������
��P2�£�III��CH3OCH3��ƽ��ת����Ϊ__________��
���𰸡�![]() �� ���� B,C �� 96%
�� ���� B,C �� 96%
��������
��1������ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮��д����ʽ��
��2���ٸ����¶ȶ�ƽ���Ӱ�������H�ķ��ţ�
��ƽ�ⳣ��Kֻ���¶��йأ�
��3�������淴Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ���Ӧ�����и����ʵ����ʵ��������ʵ���Ũ�ȼ��ٷֺ��������䣬�Լ��ɴ������һЩ���������䣬�ݴ˷������
��4����ӦΪ���������С�ķ�Ӧ������ѹǿƽ��������У�
��1.0molCH3OCH3��3.0molH2O����ʼ��2.0molCO2��6.0molH2 ��ȴﵽ��ͬ��ƽ��״̬���ݴ���ʽ���㣮
(1)ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮��,����ƽ�ⳣ��K=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)����Ϊ�¶�Խ�ߣ�CO2ת����ԽС����ƽ�������ƶ������Ը÷�Ӧ������Ϊ���ȷ�Ӧ������H��0���ʴ�Ϊ������
��Kֻ���¶�Ӱ�죬���¶Ȳ��䣬��СͶ�ϱȣ���K���䣬�ʴ�Ϊ�����䣻
(3)A.��Ӧ��ϵ��û����ɫ���壬�ʻ��������ɫʼ�ղ��䣬��A��ѡ��
B. ��Ӧǰ�������ϵ���Ͳ���ȣ���������ѹǿ���ٸı䣬��ﵽ��ƽ�⣬��Bѡ��
C. �÷�Ӧ��һ����Ӧǰ����������仯�Ŀ��淴Ӧ��������������䣬����Ӧǰ�����������仯��������ܶȲ������仯����ﵽ��ƽ�⣬��Cѡ��
D. �κ�ʱ��CH3OCH3��H2O�����ʵ���֮�Ȳ��䣬����˵����Ӧ�ﵽƽ�⣬��D��ѡ��
�ʴ�Ϊ��BC��
(4)��2CO2(g)+ 6H2(g) ![]() CH3OCH3(g) + 3H2O(l),��ӦΪ���������С�ķ�Ӧ,����ѹǿƽ��������У�CH3OCH3�����ʵ�������ͼ����ƽ��ʱCH3OCH3(g)�����ʵ�����֪P1>P2��
CH3OCH3(g) + 3H2O(l),��ӦΪ���������С�ķ�Ӧ,����ѹǿƽ��������У�CH3OCH3�����ʵ�������ͼ����ƽ��ʱCH3OCH3(g)�����ʵ�����֪P1>P2��
�ʴ�Ϊ��>��
��I��ʼ����2.0molCO2��6.0molH2��P2��ƽ�����м��㣬

ƽ��ʱCO2��ת����Ϊ4%����ʼ��1.0molCH3OCH3��3.0molH2O����ʼ��2.0molCO2��6.0molH2 ��ȴﵽ��ͬ��ƽ��״̬������Ͷ�ϴﵽƽ��ʱ��Ӧ���ת����֮��Ϊ1������P2�¢���CH3OCH3��ƽ��ת����=1-4%=96%��
�ʴ�Ϊ��96%.


 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ�п��ܺ��д�����Mg2����Al3����H����Cl��������OH���������Һ����μ���0.5 mol��L��1NaOH��Һ�����ɳ����������ͼ���NaOH��Һ�����֮��Ĺ�ϵ����ͼ��ʾ������ж�ԭ��Һ��(����)
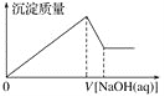
A. ��Mg2����û��Al3��
B. ��Al3����û��Mg2��
C. ��Mg2����Al3��
D. �����H����Mg2����Al3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��ԭ��������������ij���������Ԫ�أ�X��ij���⻯����ʹʪ��ĺ�ɫʯ����ֽ������Y��һ�ֺ���������Ϊ18��������Ϊ10����ͬ����Ԫ����Z�ļ����Ӱ뾶��С��W�ĵ��������õİ뵼����ϡ�����˵������ȷ����
A. ����̬�⻯���ȶ��ԣ�W >X
B. YԪ�ص����ԭ������Ϊ18
C. X2H4�ķ����м��Լ��ͷǼ��Լ���Ŀ��Ϊ4��l
D. ���Z�������Ȼ������ұ������Z
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�������ȡ����Ӧ��ʵ��װ����ͼ��ʾ������AΪ��֧�Թܸ��Ƴɵķ�Ӧ�����������¶˿���һС�ף�����ʯ���ޣ��ټ���������м��
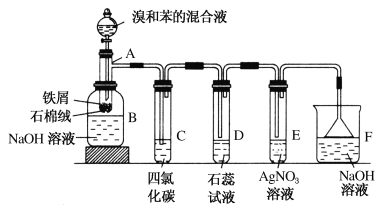
��д���пհף�
���Թ�A�еķ�Ӧ����ʽΪ_______________��
���Թ�C�����Ȼ�̼�������ǣ�____________����Ӧ��ʼ�۲�D��E���Թܣ�����������Ϊ��_________________��д��E�з�Ӧ�����ӷ���ʽ_____________________��
������������װ���У����з��������ܵ�������_____������ĸ����
(2)ʵ�����Ʊ�����������Ҫ�������£�
a. ����һ��������ŨH2SO4��ŨHNO3�Ļ���ᣬ���뷴Ӧ���У�
b. �������µĻ��������μ���һ�����ı����������Ͼ��ȣ�
c. ��55��60 ���·�����Ӧ��ֱ����Ӧ������
d .��ȥ�����ֲ�Ʒ����������ˮ��5%NaOH��Һϴ�ӣ������������ˮϴ�ӣ�
e. ������ˮCaCl2�����Ĵ��������������õ�������������
����д���пհף�
���Ʊ��������ķ�Ӧ������________��
������һ��������ŨH2SO4��ŨHNO3�Ļ����ʱ��������ע�������ǣ�___________��
�۲���d��ϴ�ӡ������������Ӧʹ�õ�������___________________��
�ܲ���d�дֲ�Ʒ��5%NaOH��Һϴ�ӵ�Ŀ����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ĸ�̱���Ϊ�̱�ʯ֮�����ǹ����鱦���繫�ϵ��Ĵ�����ʯ֮һ���仯ѧʽΪBe3Al2Si6O18���ش��������⣺
(1)��̬Beԭ�ӵĵ����Ų�ͼΪ____________����̬Alԭ�Ӻ������ռ��________�������������������ܼ���ԭ�ӹ������״Ϊ__________��
(2)Al��Si��O�ĵ�һ�������ɴ�С������Ϊ____________________��
(3)SO3��������ԭ�ӵ��ӻ�������_____��SO3���ӵĿռ乹����________��
(4)��ҵ�ϣ������ȡ�����������������ڵ�AlCl3��AlCl3�ľ���������__________����Be3Al2Si6O18дΪ�������ʽ����_____________________��
(5)LiAlH4���л��ϳ��пɽ��Ȼ���ԭ���ǻ���������Ҵ����ۡ��е���ͼ��ʾ��
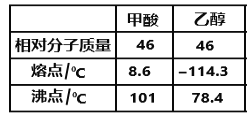
1mol��������ЦҼ���м��ı�ֵΪ________________��������Ҵ����۵����ϴ����Ҫԭ����____________________________________________��
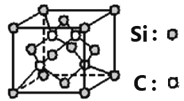
(6)̼����ľ����������ƽ��ʯ�������ṹ��ͼ��ʾ����֪��̼����ľ����ܶ�Ϊag/cm3��NA��������٤����������ֵ���þ����߳�Ϊ_____________pm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F�Ƕ���������Ԫ�أ���ԭ���������������ڶ�������AԪ��ԭ�Ӱ뾶��С��DԪ��ԭ�Ӱ뾶���B�ļ��⻯���ˮ��Һ�ʼ��ԣ�C��Eͬ���壬�γɵĻ�����Ϊ![]() ��
��![]() ���ش��������⣺
���ش��������⣺
![]() ��Ԫ�����ڱ��е�λ��Ϊ_______��
��Ԫ�����ڱ��е�λ��Ϊ_______��
![]() �Ƚ�B��C���⻯������ȶ��ԣ�_____>____��
�Ƚ�B��C���⻯������ȶ��ԣ�_____>____��![]() �ѧʽ
�ѧʽ![]()
![]() ��C��Ԫ����ɵĻ�����
��C��Ԫ����ɵĻ�����![]() ��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________
��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________
![]() д��ʵ������ȡBA3�Ļ�ѧ����ʽ__________
д��ʵ������ȡBA3�Ļ�ѧ����ʽ__________
(5)ʵ���Ҽ���BA3�ķ���_________
(6)D��F������������ˮ�������Խ�ǿ����_________(�û�ѧʽ��ʾ)
(7)�õ���ʽ��ʾ![]() _________
_________![]() _____________
_____________
(8)����˵����ȷ����__________
A��Ԫ��F�γɵĵ��ʱ�Ԫ��E�γɵĵ��ʵ��۵��
B��F��E��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸ�
C��![]() ͨ�뵽
ͨ�뵽![]() ����Һ�г��ֻ���
����Һ�г��ֻ���
D��F�⻯������Ա�E�⻯�������ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��E��һ����Ҫ���ϣ���;�㷺������һ�ֺϳ�·�����£�
![]()
��֪������Ϣ��
��A������������λ��ȡ������A��B��C��D����ʹFeCl3��Һ������ɫ��Ӧ��
��
��D��E��ʹ��ˮ��ɫ��E�к���������Ԫ����
�ش�������⣺
(1)A��������___________��A��B�ķ�Ӧ������_________________��
(2)C�еĹ�������____________��__________��д���ƣ���
(3)C��D������Ӧ�Ļ�ѧ����ʽΪ_____________________________��
(4)B�Ľṹ��ʽΪ___________��E�Ľṹ��ʽΪ______________��
(5)D��ͬ���칹���У��������������Ľṹ����________�֡����к˴Ź���������4��壬�������Ϊ3:2:2:1����________________��д�ṹ��ʽ����
�ٱ�����ֻ������ȡ����������![]() �ṹ�������ܷ���ˮ�ⷴӦ����FeCl3��Һ����ɫ���۳������⣬����������״�ṹ����ˮ�����֮һ����FeCl3��Һ������ɫ��Ӧ��
�ṹ�������ܷ���ˮ�ⷴӦ����FeCl3��Һ����ɫ���۳������⣬����������״�ṹ����ˮ�����֮һ����FeCl3��Һ������ɫ��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����д���б���
���� | ������ | ����/g | ���ʵ���/ mol | Ħ������/(g |
O2 | __ | 8.0 | __ | __ |
H2SO4 | 3.01��1023 | __ | __ | __ |
H2O | __ | __ | 0.5 | __ |
��2��147gH2SO4�����ʵ�����____��0.5molH2SO4��������____g�����к���____mol H��2 mol H2SO4�к���H2SO4������Ϊ_____��������ԭ����Ϊ____����
��3��12.4gNa2R��Na��0.4mol����Na2R��Ħ������Ϊ____��R�����ԭ������Ϊ____����R������Ϊ1.6 g��Na2R�������ʵ���Ϊ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪SO2ͨ��BaCl2��Һ����������ijͬѧ������ͼװ��̽��SO2��BaCl2��Һ��Ӧ���ɰ�ɫ�����������������ж���ȷ����
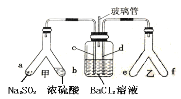
A.e��f�����е��Լ����Էֱ���Ũ��ˮ��NaOH����
B.���в�����һ��Ϊ���������壬��BaSO3����ΪBaSO4����
C.�����ܵ���������ͨ������ʹ�����е�����������ƿ�����뷴Ӧ
D.c��d�������ܶ��������BaCl2��Һ�У���֤������Ba2+��ֽӴ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com