
[��ѧ��ѡ��ѧ�뼼��]
H2O2��ˮ��Һ��һ�ֳ��õ�ɱ������
��1��H2O2�ɵĹ�ҵ�Ʒ�����PtΪ������ʯīΪ����������������Һ���ٽ�������ˮ�⡣��ѧ����ʽΪ��
![]() ��д��������������Һ��������������Ӧ����
��д��������������Һ��������������Ӧ����
������ ��
������ ��
��2��H2O2�µĹ�ҵ�Ʒ����Ƚ��һ��������ԭ���ٽ��м����ȥ����������H2O2����Ӧ����ʽΪ
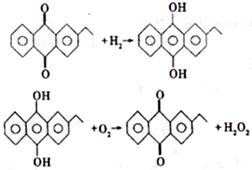
�һ������ڴ˱仯�����е������Լ���ɵĹ�ҵ�Ʒ�����¹�ҵ�Ʒ����ŵ���
��
��3��H2O2��ʵ�����Ʒ�֮һ�ǽ������������뵽ϡ�����У��÷�Ӧ�Ļ�ѧ����ʽΪ
��4��д��һ�ֶ����ⶨH2O2ˮ��Һ��H2O2�����Ļ�ѧ����ʽ
��5��д������(4)��Ӧԭ���ļ�Ҫʵ�鲽��
��1������(ʯī)��2H+��2e����H2����2�֣�����(Pt)��2HSO4����2e����S2O82����2H+��2�֣�
��2��������2�֣� �һ�������ѭ��ʹ��(���ȽϾ��õ�)��2�֣�
��3�� BaO2��H2SO4��BaSO4����H2O2��2�֣�
��4��2MnO4����5H2O2��6H����2Mn2����5O2����8H2O ��2�֣������������𰸸��֣�
��5����ȷ��ȡһ������H2O2��Һ��������
���ñ�KMnO4��Һ���еζ������������һ�Σ���Һ��Ϊ��ɫ��ֹͣ�ζ�����ȡ�����
���ظ���2��3�Σ�ȡƽ��ֵ���м��㡣 ��3�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2NH3��+CaCl2+2H2O
2NH3��+CaCl2+2H2O 2NH3��+CaCl2+2H2O
2NH3��+CaCl2+2H2O Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
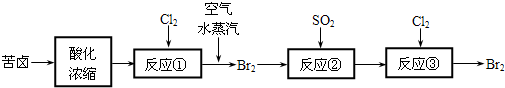
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
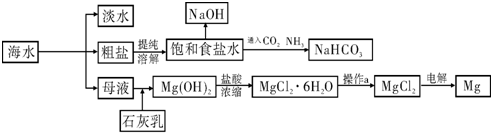
| MgO | MgCl2 | |
| �۵�/�� | 2852 | 714 |
| �е�/�� | 3600 | 1412 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com