
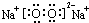 ���ʴ�Ϊ��Al4C3��
���ʴ�Ϊ��Al4C3��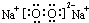 ��
�� 4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2 4NO+6H2O��
4NO+6H2O��

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| �� |
| ||
| �� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B����������Ԫ����ɵĻ����A��ij��Ԫ�ص���������Ϊ75����B��һ�ֵ���ɫ���壬C��һ����Ҫ����Դ��C��K�����ɼ��Լ����ɵķǼ��Է��ӣ�J��E�ǹ�ҵ����;�ܹ����Ҫ����ԭ�ϣ�XΪ��ɫҺ�塣(ͼ�в��ַ�Ӧ���������û���г�)

�밴Ҫ��ش�
(1)д��A�Ļ�ѧʽ H�ĵ���ʽ
(2)��Ӧ����ÿ����l mol F��ת�Ƶ��ӵ����ʵ���Ϊ
(3)��Ӧ�ڽ��е�����Ϊ
(4)��Ӧ�۵Ļ�ѧ����ʽΪ
(5)��Ӧ�ܵ����ӷ���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���㽭ʡ������ѧ����ĩ���ۻ�ѧ�Ծ��������棩 ���ͣ������
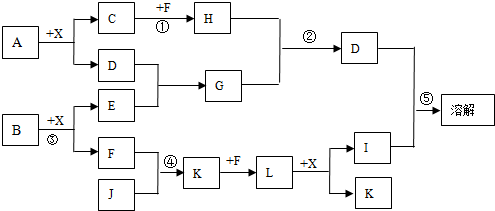
ij��֪A��B���������ֶ�����Ԫ����ɵĻ����A��ijԪ�ص���������Ϊ25%��B����ɫ��Ӧ�ʻ�ɫ��C��J��X��ͬ���ڵ�Ԫ�صļ��⻯�XΪ��ɫҺ�壬C��JΪ���壬D��һ�ֲ�����ˮ�İ�ɫ���塣��Ӧ���ɵ�ˮ������ȥ������������ͼ��ʾ�Ĺ�ϵ��

��1��д����ѧʽ��A???????? ,E??????????? ,L?????????? ��
��2���ڷ�Ӧ�٢ڢۢܢ�������������ԭ��Ӧ����???????????????????????????? ��
��3����Ӧ����ѧ����ʽΪ��?????????????????????????????????????????????? ��
��4��д���������ӷ���ʽ����Ӧ��???????????????????????????????????????? ��
G��Һ��M��Һ�ķ�Ӧ?????????????????????????????? ?????????????? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ��ͨ��ͨ����ƽ������ѧ�߿���ѧģ���Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com