
�����8�֣�
ij�л���A����NaOH��Һ��Ӧ��������к��б�������Է�������С��150�����к�̼����������Ϊ70.6%�������������Ϊ5.9%������Ϊ����
��1��A�ķ���ʽ�� ��
��2����A����NaHCO3��Һ��Ӧ�ų�CO2���壬��ṹ������ �֡�
��3����A��NaOH��Һ�ڼ���ʱ���ܽϿ췴Ӧ����1molA����1mol NaOH����A�����п��ܵĽṹ��ʽ�� ��
��4����A��NaOH��Һ�ڼ���ʱ���ܽϿ췴Ӧ����1mol A����2mol NaOH�������������A�Ľṹ������ �֣����в��ܷ���������Ӧ�����ʵĽṹ��ʽ�ǣ� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����8�֣�ij�о�С����2 L�ܱ������м�������Ļ���̿��0.2 mol NO������(T1��)�·�����Ӧ��C(s)��2NO(g) N2(g)��CO2(g)��Q(Q>0)��30 min��ﵽƽ�⣬���NOŨ��Ϊ0.04 mol/L���ش��������⣺
1���÷�Ӧ��ƽ�ⳣ������ʽK��______��T1��ﵽƽ��ʱ��N2��ƽ����Ӧ���ʣ�_______��
2��30 min�����ı�ijһ�������NO��ת���ʣ�����Ըı��������______________��
3��30 min�������¶���T2�棬�ﵽƽ���������NO��N2��CO2�Ĺ�ϵ��������____��
a��5:3:3 b��1:1:1 c��4:3:3 d��2:1:1
4������ʼʱ�ܱ����������Ϊ1 L�������������䣬�ﵽƽ�����ԭƽ����ȣ�����˵����ȷ����_________��
a��NO��ת���ʲ��� b��N2��Ũ����ԭ����2��
c����Ӧ�ų�������Ϊ0.1Q d���ﵽƽ���ʱ����ԭ����һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
( ��8��)��ij�л����к���3����CH3��1����2����CH2������д�����ϸ��������л���Ľṹ��ʽ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����У�Ϻ��У�����������ѧ�Ծ��������棩 ���ͣ������
�����8�֣�
ij�л���A����NaOH��Һ��Ӧ��������к��б�������Է�������С��150�����к�̼����������Ϊ70.6%�������������Ϊ5.9%������Ϊ����
��1��A�ķ���ʽ�� ��
��2����A����NaHCO3��Һ��Ӧ�ų�CO2���壬��ṹ������ �֡�
��3����A��NaOH��Һ�ڼ���ʱ���ܽϿ췴Ӧ����1molA����1mol NaOH����A�����п��ܵĽṹ��ʽ�� ��
��4����A��NaOH��Һ�ڼ���ʱ���ܽϿ췴Ӧ����1mol A����2mol NaOH�������������A�Ľṹ������ �֣����в��ܷ���������Ӧ�����ʵĽṹ��ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ�߶��꼶��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
( ��8��)��ij�л����к���3����CH3��1��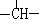 ��2����CH2������д�����ϸ��������л���Ľṹ��ʽ����������
��2����CH2������д�����ϸ��������л���Ľṹ��ʽ����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com