
��16�֣����ȷ�Ӧ�γ��µ�C-C��Ϊ�л��ϳ��ṩ��һ���µ�;�������磺
��1��������I�ķ���ʽΪ_________��������I���еĹ�����������______________��
1mol������I��ȫȼ����Ҫ����_____mol O2��
��2��������III������������ͭ��Ӧ�Ļ�ѧ����ʽΪ________________________��
��3�� ��
�� Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ��_________________________��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ��_________________________��
��4��������I�ж���ͬ���칹�壬��д������2�ַ�������������ͬ���칹��Ľṹ��ʽ��
___________��(Ҫ������FeCl3��Һ������ɫ��Ӧ���ڱ�����һ��ȡ��������2��)
��1��C8H6O3 (2��)�� �Ȼ���ȩ����2�֣��� 8��2�֣�
��2�� ��3�֣�
��3�֣�
��3��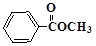 ��3�֣���4��
��3�֣���4��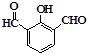 ��
�� 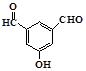 ��
�� ��
��
���������������1�����ݻ�����I�Ľṹ��ʽ�ɵøû�����ķ���ʽΪC8H6O3��������I���еĹ�����������ȩ�����Ȼ���������Iȼ�յķ���ʽ�ǣ�C8H6O3+8O2 8CO2+3H2O���ɼ�1mol�Ļ�����I��ȫȼ������8mol����������2���ڻ�����III�к���1��ȩ���������ܹ�������������ͭ��Ӧ����Ӧ������ȩ������������ͭ�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ
8CO2+3H2O���ɼ�1mol�Ļ�����I��ȫȼ������8mol����������2���ڻ�����III�к���1��ȩ���������ܹ�������������ͭ��Ӧ����Ӧ������ȩ������������ͭ�ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ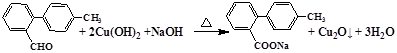 ����3��
����3�� ��
�� Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ��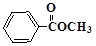 ����4��������I�ж���ͬ���칹�壬���з�������������FeCl3��Һ������ɫ��Ӧ���ڱ�����һ��ȡ��������2�ֵ�ͬ���칹��Ľṹ��ʽ��
����4��������I�ж���ͬ���칹�壬���з�������������FeCl3��Һ������ɫ��Ӧ���ڱ�����һ��ȡ��������2�ֵ�ͬ���칹��Ľṹ��ʽ��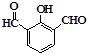 ��
�� 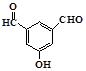 ��
�� ��
��
���㣺�����л���Ľṹ�����ʡ�ת��������ʽ��ͬ���칹�����д��֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014�㶫�����б�ҵ��ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����Ũ�Ⱦ�Ϊ0.1mol��L��1������Һ����CH3COOH����NaOH����CH3COONa������˵����ȷ����( )
A����Һ���У�c(CH3COO-)= c(H+)
B����Һ�١��ڵ������ϣ����Һ��c(CH3COO-)������Һ���е�c(CH3COO- )
C����Һ�١��ڵ������ϣ����Һ��c(CH3COO-)��c(CH3COOH) = c(Na+)
D����Һ�١��۵������ϣ����Һ��c(Na+)> c(CH3COO-)> c(H+)> c(OH-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ڻ�̬�����ԭ���У����ں�����������������������
| A������ʧȥ�ĵ���������� |
| B����������С�ĵ���������� |
| C��p�����������һ������s����������� |
| D�����������������˶��ĵ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
������ƽ���ƶ�ԭ������������ʵ��
��1��FeS������ˮ����������ϡ�����У�
��2��CaCO3������ϡ���ᣬȴ�����ڴ����У�
��3���ֱ��õ����������ˮ��0��010 mol��L-1����ϴ��BaSO4��������ˮϴ�����BaSO4����ʧ��������ϡ����ϴ�ӵ���ʧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��Ũ��ˮ���뱽�У���ˮ����ɫ��dz���������ڷ�����
| A����ѧ��Ӧ | B��ȡ����Ӧ | C���ӳɷ�Ӧ | D����ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ������������ɳ�����չ������أ����д�ʩ�����ڽ��ܼ��š�������������
�ټӿ컯ʯȼ�ϵĿ�����ʹ�ã����з����������ũҩ����Ӧ�ø�Ч�ྻ����Դת���������������սոѣ����ƹ�ʹ�ý��ܻ������ϣ���2M+N="2P+2Q" ��2P+M= Q
��M��NΪԭ�ϣ�QΪ������Ʒ�������з��ϡ���ѧ��Ӧ����ɫ������Ҫ�����
| A���٢ۢܢ� | B���ڢۢݢ� | C���٢ڢۢ� | D���ڢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����15�֣�
ij�о�С��Ϊ��̽��һ����������X����������Ԫ�أ�����ɺ����ʣ���Ʋ��������ʵ�飺
��ȡ10.80 g X�ڶ��������м�������ȫ�ֽ⣬�õ�6.40 g����1����ش��������⣺
��1��������ɫ����1�н���Ԫ�ص�ԭ�ӽṹʾ��ͼ_______ ��
д������ĵ���ʽ________ ��
��2��X�Ļ�ѧʽ��____ ���ڶ��������м���X����ȫ�ֽ�Ļ�ѧ����ʽΪ_______________ ��
��3����ɫ����2�ڿ����б�ɺ��ɫ������ԭ����________________ ��
���û�ѧ����ʽ��ʾ����
��4��һ�������£�����������1��ij�ֳɷֿ��ܷ���������ԭ��Ӧ��д��һ�����ܵĻ�ѧ��Ӧ����ʽ____________________________________________________________��
�����ʵ�鷽����֤�÷�Ӧ�IJ���_________________________________________
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��14�֣�п�̷ϵ�ؿ�������������п��̼���̣��乤�����̷�ΪԤ����������п�����ߡ�̼���������������֡���������̼���̵Ĺ����������£�
��ش��������⣺
��1��п�̷ϵ�صĻ��չ����У���ѭ�������Ƕȿ��ǣ�������������Դ������________��
��2��ԭ���̷۴�Ʒ����Ҫ�ɷ�ΪMnO2��̼������ʱһ����MnO2����ԭ����һ��
�ֱ�̼��ԭ��MnO2����ԭΪһ�����̡���֪��
д��C(s)��MnO2(s)����ΪCO(g)���Ȼ�ѧ����ʽ��________��
��3��50-55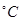 ʱ����MnSO4��ĸҺ�м�������NH4HCO3����Ӧ�Ļ�ѧ����ʽΪMnSO4��
ʱ����MnSO4��ĸҺ�м�������NH4HCO3����Ӧ�Ļ�ѧ����ʽΪMnSO4��
��4����֪�������ӳ���ʱ��pH��Χ��Fe3+��2.7~3.7��Mn2+��8.6~10.1��Fe2+��7.6~9.6��������г�ȥ�ķ������������������¼���_________,��Fe2������ΪFe3����Ȼ���ٽ�pH����_______��ʹFe3��������ȫ��
��5����ƷMnCO3�����������������Һ��������Һ�ɵö������̣�д�������ĵ缫��Ӧʽ��_____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com