
25 ��ʱ������ƽ�ⳣ����
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | 1.8��10��5 | K1 4.3��10��7 K2 5.6��10��11 | 3.0��10��8 |
�ش��������⣺
(1)���ʵ���Ũ��Ϊ0.1 mol��L��1�������������ʣ�a.Na2CO3��b.NaClO��c.CH3COONa��d.NaHCO3��pH�ɴ�С��˳����______________(����)��
(2)������0.1 mol��L��1��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ����С����________������������������������������������
A��c(H��) B. C��c(H��)��c(OH��) D.
C��c(H��)��c(OH��) D.
(3)���Ϊ10 mL pH��2�Ĵ�����Һ��һԪ��HX��Һ�ֱ��ˮϡ����1 000 mL��ϡ����pH�仯��ͼ����HX�ĵ���ƽ�ⳣ��________(������������������������С����)�����ƽ�ⳣ����������__________________________________________��ϡ�ͺ�HX��Һ��ˮ���������c(H��)________������Һˮ�������c(H��)(������������������������С����)�����ǣ�____________________________________��

(4)25 ��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH��6������Һ��c(CH3COO��)��c(Na��)��________(��ȷ��ֵ)��
��(1)a��b��d��c ��(2)A��(3)���ڡ�ϡ����ͬ������HX��Һ��pH�仯��CH3COOH��Һ�Ĵ�����ǿ������ƽ�ⳣ�����ڡ�HX����ǿ��CH3COOH��ϡ�ͺ�c(H��)С��CH3COOH��Һ�е�c(H��)�����Զ�ˮ��������������
(4)9.9��10��7 mol��L��1
����������(1)�۲����ƽ�ⳣ����֪����ΪCH3COOH>H2CO3>HClO>HCO3��������Щ��ʧȥ�����Ӻ�ˮ������ȴ�����෴�����Եó�pH��С˳��Ϊa��b��d��c��(2)������������ʣ�ϡ�ͺ����̶�����CH3COOH��CH3COO����H��Ũ��ȴ��Ҫ��С����c(OH��)ȴ������ģ���CH3COOHŨ�ȼ�����࣬���A����ȷ��(3)ϡ����ͬ������HX��Һ��pH�仯��CH3COOH��Һ�Ĵ�����ǿ������ƽ�ⳣ����HX�ĵ��볣������CH3COOH�ĵ��볣����HX����ǿ��CH3COOH��ϡ�ͺ�c(H��)С��CH3COOH��Һ�е�c(H��)�����Զ�ˮ����������������(4)���ݵ���غ��c(CH3COO��)��c(Na��)��c(H��)��c(OH��)��10��6 mol��L��1��10��8 mol��L��1��9.9��10��7 mol��L��1��


 �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� �߿�ģ������2��ϰ���������棩 ���ͣ������
��Ȼ��������Ҫ����Դ��Ҳ����Ҫ�Ļ���ԭ�ϣ�����Ҫ�ɷ��Ǽ��顣
��1��������һ�������¿����ɣ�
A��̼�����ӣ�CH3-������������ B��̼�����ӣ�CH3-��
C��������CH3�� D��̼ϩ�� ��
��
���������У�����Ϊ120������________��������ţ�
����̼�����ӣ�CH3-����Ϊ�ȵ������һ�ַ��ӵĽṹʽΪ_________________________________________________________��
��2�������ش����Ŀ�ȼ���������ˮ��������ڿ�ȼ����˵������ȷ����________��
A�����������ˮ���Ӿ��Ǽ��Է���
B����ȼ���м��������ˮ���Ӽ����������
C����ȼ������ԭ�Ӿ���
D�����������ˮ�����е���������s��sp3�������ص����ɵ�
��3���ڸ����£�����ɻ�ԭCuO�õ�Cu2O��
��Cu���ĺ�������Ų�ʽΪ____________��
��Cu2O����ľ����ṹ��ͼ��ʾ���������������������ӷ���Ϊ________��

��4��һ�������£�������ˮ����������H2��CO���������ɵ�������������������������֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� �߿�ģ������1��ϰ���������棩 ���ͣ�ѡ����
�����йػ�ѧ�����ʾ��ȷ���ǣ���������
A��CO2�ĵ���ʽ��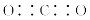
B��������Ϊ43��������Ϊ56��Tcԭ�ӣ�
C��Cl���Ľṹʾ��ͼ��
D����������Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��8���绯ѧ��ϰ���������棩 ���ͣ������
��������ӵ�ز���Li2MSiO4(MΪFe��Co��Mn��Cu��)��һ�ַ�չDZ���ܴ�ĵ�ص缫���ϡ���ҵ�Ʊ�Li2MSiO4�����ַ�����
����һ�����෨��2Li2SiO3��FeSO4 Li2FeSiO4��Li2SO4��SiO2��
Li2FeSiO4��Li2SO4��SiO2��
���������ܽ�����������CH3COOLi��Fe(NO3)3��Si(OC2H5)4���Լ� ����
���� ������
������ Li2FeSiO4��
Li2FeSiO4��
(1)���෨���Ʊ�Li2FeSiO4���̲��ö����������գ���ԭ����____________________________________________________��
(2)�ܽ�?�������У������Һ���н������ɵķ�����________�������У�����1 mol Li2FeSiO4��������ת�Ƶ��ӵ����ʵ���Ϊ________mol��
(3)��Li2FeSiO4��Ƕ��Li��ʯīΪ�缫���ϣ���﮵ĵ������������ʣ����ɵ�ص��ܷ�ӦʽΪLi��LiFeSiO4 Li2FeSiO4����õ�صĸ�����________�����ʱ��������Ӧ�ĵ缫��ӦʽΪ________��
Li2FeSiO4����õ�صĸ�����________�����ʱ��������Ӧ�ĵ缫��ӦʽΪ________��
(4)ʹ��(3)��װ�ĵ�ر�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��8���绯ѧ��ϰ���������棩 ���ͣ�ѡ����
��ҵ�Ͽ������̿�(��Ҫ�ɷ�ΪMnO2)����п��(��Ҫ�ɷ�ΪZnS)��ȡ�ɵ���������MnO2��Zn���乤���������£�

����˵����ȷ����(����)��
A������ʱ��MnO2����ԭ��
B������������������������
C��ԭ���������ѭ��ʹ��
D���ڵ��ص�����������MnO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��7��ˮ��Һ�е�����ƽ����ϰ���������棩 ���ͣ�ѡ����
25 ��ʱ��0.1 mol Na2CO3�����������õ�һ�����Ϊ1 L����Һ����Һ�в�������pH�Ĺ�ϵ��ͼ��ʾ�������й���Һ������Ũ�ȹ�ϵ��������ȷ����(����)��
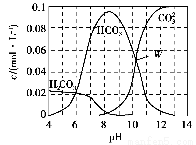
A��W����ʾ����Һ�У�c(Na��)��c(H��)��2c(CO32��)��c(OH��)��c(Cl��)
B��pH��4����Һ�У�c(H2CO3)��c(HCO3��)��c(CO32��)<0.1 mol��L��1
C��pH��8����Һ�У�c(H��)��c(H2CO3)��c(HCO3��)��c(OH��)��c(Cl��)
D��pH��11����Һ�У�c(Na��)>c(Cl��)>c(CO32��)>c(HCO3��)>c(H2CO3)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��6����ѧ��Ӧ���ʺͻ�ѧƽ����ϰ���������棩 ���ͣ������
��һ�ݻ�������ܱ������г���һ����A��B��������Ӧ��xA(g)��2B(s) yC(g)����H��0����һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
yC(g)����H��0����һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺

(1)��A��Ũ�ȱ仯��ʾ�÷�Ӧ0��10 min�ڵ�ƽ����Ӧ����v(A)��________��
(2)����ͼʾ��ȷ��x��y��________��
(3)0��10 min������ѹǿ________(�������������������������С��)��
(4)�Ʋ��10 min�������߱仯�ķ�Ӧ����������________����16 min�������߱仯�ķ�Ӧ����������________��
����ѹ��������A��Ũ�ȡ�������C������������ �����¡����Ӵ���
(5)��ƽ������ƽ�ⳣ��ΪK1��ƽ������ƽ�ⳣ��ΪK2����K1________K2(������������������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��5����ѧ��Ӧ��������ϰ���������棩 ���ͣ�ѡ����
��֪��Ȳ�뱽������ȫȼ�յ��Ȼ�ѧ����ʽ���£�
��C2H2(g)��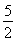 O2(g)�D��2CO2(g)��H2O(l) ��H����1 300 kJ��mol��1
O2(g)�D��2CO2(g)��H2O(l) ��H����1 300 kJ��mol��1
��C6H6(g)��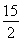 O2(g)�D��6CO2(g)��3H2O(l) ��H����3 295 kJ��mol��1
O2(g)�D��6CO2(g)��3H2O(l) ��H����3 295 kJ��mol��1
����˵����ȷ����(����)��
A��1 mol C2H2(g)��ȫȼ��������̬ˮʱ���ȴ���1 300 kJ
B��1 mol C6H6(l)��ȫȼ������Һ̬ˮʱ���ȴ���3 295 kJ
C����ͬ�����£���������C2H2(g)��C6H6(g)��ȫȼ�գ�C6H6(g)���ȸ���
D��C2H2(g)��������C6H6(g)�Ĺ������ڷ��ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��1�����ʵ�������ʺͷ�����ϰ���������棩 ���ͣ������
�輰�仯����������ִ������ķ�չ��������ס���ش������й����⣺
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
��������Ͽˮ���ӡ���ʯӢ���ά�����մ�����
����ͨ����������̫���ܵ��
A���٢ڢ� B���ۢܢ� C���ڢۢ� D���٢ۢ�
(3)�����£�SiCl4ΪҺ̬���е�Ϊ57.6 �����ڿ�����ð�������Ʊ��ߴ��ȹ���м����SiCl4������Һ̬���ʣ���Ҫ�õ��ߴ���SiCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��
___________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com