
��������Դ�ı��⣬�̲��ŷḻ�Ļ�ѧԪ�أ����ȡ��塢�⡢þ�ȡ���Ҫ��ش��������⣺
��1������֤����ȵ��������ǿ�����ӷ�Ӧ����ʽΪ____________________________��
��2���Ӻ���������ȡ�ĵⵥ�ʣ���������һ�������ʣ�ͨ�����ü��ȵķ�����ȥ���ʣ������������Ҫ���õ������е�___________��Ҳ����ͨ����ȡ��Һ�ķ�����ȡ�⣬ʵ�����з�Һʱ����Һ©���е��ϲ�Һ��Ӧ�ӷ�Һ©����_______����ϡ����¡����ڵ�����
��3����ȼ��þ�������ڶ�����̼�����м���ȼ�գ��÷�Ӧ�Ļ�ѧ����ʽΪ__________��
��1��2I����Br2��I2��2Br�� ��2�����ʵ��������� �� ��3��2Mg��CO2 2MgO��C
2MgO��C
���������������1���������ܰѵ������������ɵ��ʵ⣬����������ԭ��Ӧ����������������ǿ����������������Կ�֪����ȵ�������ǿ����Ӧ�����ӷ���ʽΪ2I����Br2��I2��2Br����
��2�����ڵⵥ��������������ͨ�����ü��ȵķ�����ȥ���ʵ��е����ʡ���Һʱ��Һ©���е��ϲ�Һ��Ӧ�ӷ�Һ©�����Ͽڵ������²�Һ���С��������
��3��þ������CO2��ȼ����������þ��̼����Ӧ�Ļ�ѧ����ʽΪ2Mg��CO2 2MgO��C��
2MgO��C��
���㣺����������ԭ��Ӧ��Ӧ�á����ʵ�����ʡ���Һ�����Լ�þ��CO2��ȼ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ԭ��Ӧ��ʵ���ϰ��������ͻ�ԭ�������̡�������һ����ԭ���̵ķ�Ӧʽ��NO��4H����3e��=NO����2H2O��KMnO4��Na2CO3��Cu2O��Fe2(SO4)3���������е�һ������(��)��ʹ������ԭ���̷�����
��1��д������ƽ��������ԭ��Ӧ�ķ���ʽ��__________________________________��
��2����Ӧ������������______________��______________�����ʡ�
��3����Ӧ��������0.2 mol���壬��ת�Ƶĵ��ӵ����ʵ�����________ mol��
��4����1 mol����ijŨ�����ᷴӦʱ������ԭ��������ʵ������ӣ�ԭ����________________________________________________________________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����������Ҫ�Ĺ�ҵԭ�ϡ�
�ٿ��������������Ũ����������� ��
��ij���᳧����β��NO2�ķ����ǣ���������ʱ��H2��NO2��ԭΪN2��
��֪��2H2(g) + O2(g) �� 2H2O(g) ��H����483 kJ��moL��1
N2(g) + 2O2(g) �� 2NO2(g) ��H����68 kJ��moL��1
��H2��ԭNO2����ˮ�������Ȼ�ѧ����ʽ�ǣ�
��
��2��ij�о�С����CaCl2��H2Ϊԭ���Ʊ�+1��Ca�Ļ����������ֻ�м������ֻ�����о����֣������������иơ���Ԫ�ص����������ֱ�Ϊ52.29%��46.41%���������ҵ�ˮ��Һ�����ԡ���ش��������⣺
���ҵĻ�ѧʽΪ ������ˮ��Ӧ�ɵ�H2���仯ѧ����ʽ�ǣ� ��
��д����CaCl2ͨ�����Ϸ�Ӧ�Ʊ�CaCl�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ƫ��������N2O4�dz��õĻ���ƽ��������߷������»�ѧ��Ӧ��
(CH3)2NNH2 (l)��2N2O4 (l)��2CO2 (g)��3N2(g)��4H2O(g) ��I��
(1)��Ӧ��I������������_______��
(2)����к��г��ֺ���ɫ���壬ԭ��Ϊ��N2O4 (g)  2NO2 (g) ���� һ���¶��£���Ӧ�����ʱ�Ϊ��H���ֽ�1 mol N2O4 ����һ��ѹ�ܱ������У�����ʾ��ͼ��ȷ����˵����Ӧ�ﵽƽ��״̬����________��
2NO2 (g) ���� һ���¶��£���Ӧ�����ʱ�Ϊ��H���ֽ�1 mol N2O4 ����һ��ѹ�ܱ������У�����ʾ��ͼ��ȷ����˵����Ӧ�ﵽƽ��״̬����________��
������ͬ�¶��£�������Ӧ�������Ϊ1L�ĺ����ܱ������н��У�ƽ�ⳣ��________����������䡱��С��������Ӧ3s��NO2�����ʵ���Ϊ0.6mol����0��3s�ڵ�ƽ����Ӧ����v��N2O4����________mol��L-1��s-1��
(3)NO2���ð�ˮ��������NH4NO3��25��ʱ����amol NH4NO3����ˮ����Һ�����ԣ�ԭ����_____
_______________________________________________�������ӷ���ʽ��ʾ���������Һ�μ�bL��ˮ����Һ�����ԣ���μӰ�ˮ�Ĺ����е�ˮ�ĵ���ƽ�⽫______������������������ƶ������μӰ�ˮ��Ũ��Ϊ_______mol��L-1����NH3��H2O�ĵ���ƽ�ⳣ��ȡKb��2��10-5 mol��L-1��������Һ�������bL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���û��ϼۺ���������Ʋ����ʵ������ǻ�ѧ�о�����Ҫ�ֶΡ�
��1���ӻ��ϼ۵ĽǶȿ���Ԥ�����ʵ����ʡ�
��SO2������___________������ţ���ͬ����
A��ֻ�������� B��ֻ�л�ԭ�� C���������������л�ԭ��
�ڽ�SO2ͨ������KMnO4��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ��������__________��
A��S2�� B��S C��SO32�� D��SO42��
��2�������ʷ���ĽǶȿ����Ʋ����ʵ����ʡ�������MgO��Al2O3��Fe2O3��SiO2��ɵ�ij�����������
������Al2O3����_______�����MgO��Fe2O3����_________���������ԡ��������ԡ������ԡ�����
�ڽ��������ڹ����������У����ˣ���������Ҫ�ɷ���_________��������Һ�м���NaOH��Һ�����������ˣ������е���Ҫ�ɷ���_________��
������������ֱ�����ڹ�����NaOH��Һ�У��������ķ�Ӧ�Ļ�ѧ����ʽ��______________________��������д����һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(4��) ʵ���ҿ����ø�����غ�Ũ���ᷴӦ��ȡ��������Ӧ�Ļ�ѧ����ʽ����
2KMnO4+16HCl(Ũ) = 2KCl+2MnCl2+5Cl2��+8H2O
��1���ڸ÷�Ӧ�У���ԭ���� ��
��2�����ڷ�Ӧ�������˱����2.24L�����������ת�Ƶĸ����� NA��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������Ļ������ڹ�����ռ����Ҫ��λ���ϳɰ���ҵ�У��ϳ�����ÿ����2 mol NH3���ų�92.4 kJ������
��1������ʼʱ�������ڷ���2 mol N2��6 mol H2����ƽ���ų�������ΪQ����Q_____184.8kJ���>������<����=���� �� һ�������£����ܱպ��ݵ������У��ܱ�ʾ��Ӧ�ﵽ��ѧƽ��״̬����____________��
a��3v��(N2)=v��(H2) ������ b��2v��(H2)= v��(NH3)
c����������ܶȱ��ֲ��� �� d��c(N2)��c(H2)��c(NH3)=1��3��2
��ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO(NH2)2]����Ӧ�Ļ�ѧ����ʽΪ2NH3 (g)+ CO2 (g)  CO(NH2)2 (l) + H2O (l)��
CO(NH2)2 (l) + H2O (l)��
��2����һ���¶Ⱥ�ѹǿ�£���ԭ�����е�NH3��CO2�����ʵ���֮�ȣ���̼�ȣ�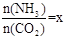 ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����___________��
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����___________��
��3��ͼ�е�B�㴦��NH3��ƽ��ת����Ϊ_______��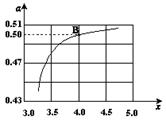
��֪��3Cl2+2NH3��N2+6HCl �D�D�� 3Cl2+8NH3��N2+6NH4Cl �D�D��
��4����ɲ���ƽ����������ԭ��Ӧ����ʽ���ٱ������ת�Ƶķ������Ŀ��
12Cl2+15NH3�� �D�D��
��5����Ӧ���еĻ�ԭ���� ����ԭ������ ��
��6�������۷�Ӧ���������9.408L����״����������������������ʵ����� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I��1���ڵ��۵⻯����Һ��ͨ�����������������ῴ����Һ����ɫ����Ӧ�����ӷ���ʽ�� ��
��2���ڵ�͵����γɵ���ɫ��Һ��ͨ��SO2���壬������ɫ����ʧ����Ӧ�����ӷ����� ��
��3���Աȣ�1���ͣ�2��ʵ�����õĽ������Cl ��I
��I SO2����ԭ����ǿ����˳������Ϊ ��
SO2����ԭ����ǿ����˳������Ϊ ��
II ��4�� ��ȥ�����л������۵��Լ��� �����ӷ���ʽΪ
��5�� 1mol����������2mol̼�����ƹ����Ϻ����ܱ������м��ȳ�ַ�Ӧ���ų��������ʺ���ȴ�������Ĺ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��ȡ����Ҳ����Ũ������������Ϊԭ�ϣ��仯ѧ����ʽΪ
2KMnO4+16HCl(Ũ)��2MnCl2+2KCl+5Cl2��+8H2O����ش��������⣺
��1����˫���ŷ�����û�ѧʽ����ת�Ƶķ�������Ŀ��
2KMnO4+16HCl(Ũ)��2MnCl2+2KCl+5Cl2��+8H2O��
��2����Ӧ�б���ԭ��Ԫ��Ϊ ��д���ƣ�����״���µ�����112 L����ʱ����Ӧ��ת�Ƶĵ�����ĿΪ ��
��3������4 molHCl��������������ɱ�״���µ����� L��
��4������1.58g������غ�100mL10moL/LŨ�����ַ�Ӧ������������ӷ���������Һ�����ǰ��仯������Ӧ���յ�ʱ���������ɫ��ȫ��ȥ����������HCl�����ʵ���
Ϊ mol������Ӧ�����Һȡ��10mL��������������������Һ���ɵõ����������ʵ���Ϊ mol��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com