
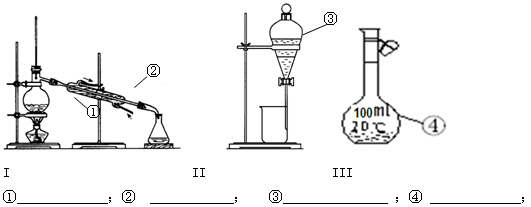


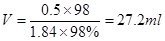 �����ѡ��50ml��Ͳ������ѡB��
�����ѡ��50ml��Ͳ������ѡB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����״���£�4.48 L N2��O2�Ļ������������ԭ����Ϊ0.4NA |
| B�������£�1.0 L 1.0 mol��L-1NaAlO2��Һ�к��е���ԭ����Ϊ2NA |
| C���ڱ�״���£�22.4 L������22.4 L���������е�ԭ������Ϊ2NA |
| D��0.1 mol Fe�μ�������ԭ��Ӧ��ת�Ƶĵ�����Ŀһ����0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��δϴ���ܽ�NaCl���ձ� |
| B��������ƿ����Һʱ��������Һ�彦�� |
| C������ʱ���ӿ̶��� |
| D������ƿ������ϴ�Ӻ������������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1 mol����������������������ΪNA |
| B����ϩ��C2H4���ͻ����飨C3H6����ɵ�28 g��������к���2NA����ԭ�� |
C��1mol Na2O2�������NA��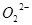 |
| D������0.1 mol�Ȼ����ı�����Һ�μӵ���ˮ�п���ȡ0.1NA�� Fe(OH)3������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��7.8g Na2O2�����к��е�������������Ϊ0.3NA |
| B��25��ʱ��1L pH=13��NaOH��Һ����ˮ���������OH������ԼΪ1��10��13NA |
| C��4.4g CO2�����еijɼ�������ԼΪ0.8NA |
| D���ڱ�״���£���������N2��CO�ķ�������ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����״���£�2.24LH2���еķ�����Ϊ0.1 NA |
| B����״���£�1 NA H2O����ռ�е����ԼΪ22.4L |
| C�����³�ѹ�£�1.06g Na2CO3���е���ԭ����ĿΪΪ0.03 NA |
| D�����ʵ���Ũ��Ϊ0.5mol /L��AlCl3��Һ�У�Cl-Ũ��Ϊ1.5mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com