
��10�֣��������ʵ���Ũ��Ϊamol��L-1�ı�NaOH��Һȥ�ζ�VmL��������ʵ���Ũ�ȣ�����д���пհף�
��1����ʽ�ζ���������ˮϴ��������Ӧ�ý��еIJ�����________________________��
��2���ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע�� ��ѡ��ס����ҡ����С�
��3����ͼ�Ǽ�ʽ�ζ�����Һ���ڵζ�ǰ��Ķ����� c(HCl)=_______________mol��L-1��
��4�����ڵζ�ǰ�ζ��ܼ��첿���������ݣ��ζ���ζ��ܼ��첿��������ʧ����ζ���HCl���ʵ���Ũ�Ȼ�ƫ____________(���С��)��
��5��ȡ20.00 mL�������������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�ҺNaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������¡�
| ʵ���� | NaOH��Һ��Ũ�ȣ�mol��L-1�� | �ζ����ʱ�� NaOH��Һ����������mL�� | ��������������mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ����
ʵ����| (V2-V1)a |
| V |
| (V2-V1)a |
| V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������ʵ���Ũ��Ϊa mol?L-1�ı�NaOH��Һȥ�ζ�V mL��������ʵ���Ũ�ȣ�����д���пհף�
�������ʵ���Ũ��Ϊa mol?L-1�ı�NaOH��Һȥ�ζ�V mL��������ʵ���Ũ�ȣ�����д���пհף�| a ��(V2-V1) |
| 2V |
| a ��(V2-V1) |
| 2V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1�������й�������ʹ�ã���ʵ�ֵ���
��1�������й�������ʹ�ã���ʵ�ֵ���| (V2-V1)a |
| V |
| (V2-V1)a |
| V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| a(V2-V1) |
| V |
| a(V2-V1) |
| V |
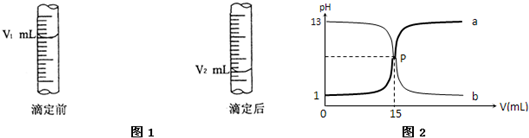
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| a(V2-V1) |
| V |
| a(V2-V1) |
| V |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com