



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����һ����NaOHϡ��Һ��ϡ���ᷴӦ��õ��к������ݣ�������һ����ϡH2SO4��NaOHϡ��Һ��Ӧ�ķ�Ӧ�� |
| B���÷е������Ʋ��ܷ�������ķ���������Һ��������з��� |
| C���÷�Ӧ�����ݵĴ�С�жϲ�ͬ��Ӧ�ķ�Ӧ���ʴ�С |
| D����ԭ�ӣ������ӣ��뾶�����ƶ�ͬ���ڻ�ͬ����ijЩԭ�ӣ������ӣ������Ի�ԭ�Ե�ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺 HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______
HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______ __________________________________��
__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʵ�飨�����Һ�� | A | B | C | D | E | F |
| 4mol/L ����/mL | 60 | V1 | V2 | V3 | V4 | V5 |
| ����CuCl2��Һ/mL | 0 | 1.0 | 5.0 | 10 | V6 | 40 |
| H2O/mL | V7 | V8 | V9 | V10 | 20 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
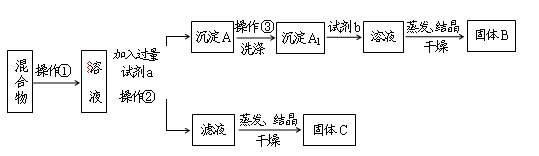
 ��Һ��ȥ
��Һ��ȥ ���Ȼ������ɫ����
���Ȼ������ɫ����| A���٢ڢۢ� | B���ڢܢݢ� | C���٢ۢݢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢܢ� |
| B���٢ڢۢ� |
| C���ڢܢݢ� |
| D���ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���٢ڢ� | C���ۢܢ� | D���ݢߢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
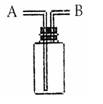
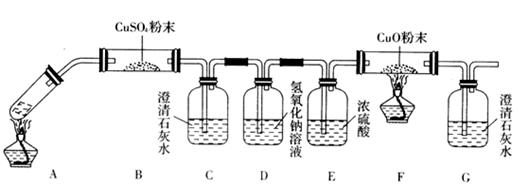
| A��ƿ��ʢ��ˮ����B�ڽ���������ˮ���ռ�H2 ? | B��ƿ��ʢ����Ũ���ᣬ��A�ڽ���������CO2 ? | C����B�ڽ��������ſ������ռ�NO2 ? | D��ƿ��װ��ˮ��A�������ܲ�������Ͳ�У���B�ڽ���������ˮ����������O2����� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com