
�ȡ��塢���±��Ԫ����Ҫ���Ժ�ˮ���ܶຣ��ֲ���ж����д����ĵ⣬��Ӧ���ǴӺ��������ȡ�����Ҫ��Ӧ����Ӧ���Ǵ�������ʯ����ȡ�����Ҫ��Ӧ��
��2NaI��MnO2��3H2SO4===2NaHSO4��MnSO4��2H2O��I2��
��2NaIO3��5NaHSO3===2Na2SO4��3NaHSO4��H2O��I2��
�����й�˵����ȷ����(����)
A��NaI��NaIO3��һ���������ܷ�Ӧ����I2
B��I2�ڷ�Ӧ�����ǻ�ԭ����ڷ�Ӧ��������������
C��������Ӧ�����ɵ�����I2ʱת�Ƶ��������
D�������ԣ�MnO2��IO![]() ��I2��SO
��I2��SO![]() ��Mn2��
��Mn2��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����Ʊ���һ��ѧ³�ư� ³�ư� ���ͣ�022
��ͺ�ˮ����
1����Ĵ���
������99������Ԫ����Br������ʽ�����ں�ˮ�У��屻��Ϊ________�����Ǻ�ˮ�еij���Ԫ�أ�
2��±��Ԫ��
F��Cl��Br��I��ԭ�ӽṹ��Ԫ��������һ���������ԣ�ͨ��Ϊ±��Ԫ�أ�
3���嵥�ʺ͵ⵥ�ʵ���������
ʵ��1��

ʵ��2�������Ȳ���Һ��ֱ�ӱ���˹��塪��������
����Һ̬�����ӷ�������������ˣ�������ʢ����Լ�ƿ���һЩˮ����ֹ��ӷ��������������ܱձ��森
���ڵ������������Ա��뽫���������������ܱձ��森
�������������ʣ������ڵ����ȡ�ʹӻ�����еķ��룬��ȡָ�Ƶȣ�
4���ȡ��塢�ⵥ�������Ե�ǿ��
ʵ�鷽����ʵ���¼��
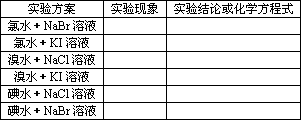
��������1�������ԣ�Cl2��Br2��I2(��S)
��������2��Cl2�ɴ��廯�����Һ���Br2�û�������
2Br����Cl2![]() Br2��2Cl��
Br2��2Cl��
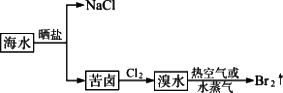
5����ˮ����Ĺ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������人�����ص���ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ���ѡ��
�ȡ��塢���±��Ԫ����Ҫ���Ժ�ˮ���ܶຣ��ֲ���ж����д����ĵ⣬��Ӧ���ǴӺ��������ȡ�����Ҫ��Ӧ����Ӧ���Ǵ�������ʯ����ȡ�����Ҫ��Ӧ��
2NaI��MnO2��3H2SO4===2NaHSO4��MnSO4��2H2O��I2��
2NaIO3��5NaHSO3===2Na2SO4��3NaHSO4��H2O��I2�������й�˵����ȷ���� (����)
| A��NaI��NaIO3��һ���������ܷ�Ӧ����I2 |
| B��I2�ڷ�Ӧ�����ǻ�ԭ����ڷ�Ӧ�������������� |
| C��������Ӧ�����ɵ�����I2ʱת�Ƶ�������� |
D�������ԣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������人�����ص���ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�ȡ��塢���±��Ԫ����Ҫ���Ժ�ˮ���ܶຣ��ֲ���ж����д����ĵ⣬��Ӧ���ǴӺ��������ȡ�����Ҫ��Ӧ����Ӧ���Ǵ�������ʯ����ȡ�����Ҫ��Ӧ��
2NaI��MnO2��3H2SO4===2NaHSO4��MnSO4��2H2O��I2��
2NaIO3��5NaHSO3===2Na2SO4��3NaHSO4��H2O��I2�������й�˵����ȷ���� (����)
A��NaI��NaIO3��һ���������ܷ�Ӧ����I2
B��I2�ڷ�Ӧ�����ǻ�ԭ����ڷ�Ӧ��������������
C��������Ӧ�����ɵ�����I2ʱת�Ƶ��������
D�������ԣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com