
| ʵ�� ��� |
HA���ʵ��� Ũ��/��mol?L-1�� |
NaOH���ʵ��� Ũ��/��mol?L-1�� |
��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |


 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | HA��Ũ��/��mol?L-1�� | NaOH��Ũ��/��mol?L-1�� | �����Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | c | 0.2 | pH=7 |
| �� | 0.1 | 0.1 | pH��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2013?����һģ�������£���ijһԪ��HA��NaOH��Һ�������ϣ�ʵ����Ϣ���£�
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HA��Ũ��/��mol/L�� | NaOH��Ũ��/��mol/L�� | �����Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
��ش�
��1��������������ʵ���������Ӽ��������������a 7(�����������������)��HAΪ���
��2���������������ʵ���������������������������������һ���������
A.����Һ�����ʵ���Ũ�ȴ��ڼ���Һ
B.����Һ��H+��Ũ�ȴ��ڼ���Һ��OH����Ũ��
C.����Һ�����ʵ���Ũ��С�ڼ���Һ
D.����Һ��H+��Ũ��С�ڼ���Һ��OH����Ũ��
E.������Һ�����ʵ���Ũ�����
��3���ӱ���ʵ����������HA�� �ᣨ�ǿ������������ ����ʱ10mL0.5mol/LNaA��Һ��6mL1mol/L�����Ϻ���Һ�г�OH����ĸ�����Ũ���ɴ�С˳��Ϊ ��
��4������ʵ�����û����Һ����ˮ���������c(OH��)= mol/L��
��5������HA��һԪ���ᣬ��������CaA2��ˮ�д����ܽ�ƽ�⣺CaA2(s) ![]() Ca2++2A-
Ca2++2A-
��H��0��һ���¶���CaA2������Һ��Ksp=c(Ca2+)c2(A2-)Ϊһ������
���¶�����ʱ��Ksp �����������С�����䡱����
�ڲ��25��ʱ��CaA2��KspΪ4.0��10-11�������½�10g CaA2����Ͷ��100mLCaCl2��Һ�У���ֽ�������й���ʣ�࣬�����Һ��c(Ca2+)=0.1mol/L������Һ��c(A2-)= ��
��ƽ�ⳣ�������˷����ϵ�Ŀ��淴Ӧ�ڸ������¶��½��еij̶ȣ�����ͬһ�����͵ķ�Ӧ��ƽ�ⳣ��Խ������Ӧ���еij̶�Խ��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
����������̼��ʹ�����ĵ���ƽ�ⳣ����д����������������������Ӧ�����ӷ���ʽ��
(1)������������ͨ�뵽������̼������Һ��___ _____________��
(2)������̼���ư���1�U1�����ʵ���֮��ǡ�÷�Ӧ_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HA��Ũ��/��mol/L�� | NaOH��Ũ��/��mol/L�� | �����Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
��ش�
��1��������������ʵ���������Ӽ��������������a 7(�����������������)��HAΪ���
��2���������������ʵ���������������������������������һ���������
A.����Һ�����ʵ���Ũ�ȴ��ڼ���Һ
B.����Һ��H+��Ũ�ȴ��ڼ���Һ��OH����Ũ��
C.����Һ�����ʵ���Ũ��С�ڼ���Һ
D.����Һ��H+��Ũ��С�ڼ���Һ��OH����Ũ��
E.������Һ�����ʵ���Ũ�����
��3���ӱ���ʵ����������HA�� �ᣨ�ǿ������������ ����ʱ10mL0.5mol/LNaA��Һ��6mL1mol/L�����Ϻ���Һ�г�OH����ĸ�����Ũ���ɴ�С˳��Ϊ ��
��4������ʵ�����û����Һ����ˮ���������c(OH��)= mol/L��
��5������HA��һԪ���ᣬ��������CaA2��ˮ�д����ܽ�ƽ�⣺CaA2(s) ![]() Ca2++2A-
Ca2++2A-
��H��0��һ���¶���CaA2������Һ��Ksp=c(Ca2+)c2(A2-)Ϊһ������
���¶�����ʱ��Ksp �����������С�����䡱����
�ڲ��25��ʱ��CaA2��KspΪ4.0��10-11�������½�10g CaA2����Ͷ��100mLCaCl2��Һ�У���ֽ�������й���ʣ�࣬�����Һ��c(Ca2+)=0.1mol/L������Һ��c(A2-)= ��
��ƽ�ⳣ�������˷����ϵ�Ŀ��淴Ӧ�ڸ������¶��½��еij̶ȣ�����ͬһ�����͵ķ�Ӧ��ƽ�ⳣ��Խ������Ӧ���еij̶�Խ��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
����������̼��ʹ�����ĵ���ƽ�ⳣ����д����������������������Ӧ�����ӷ���ʽ��
(1)������������ͨ�뵽������̼������Һ��___ _____________��
(2)������̼���ư���1�U1�����ʵ���֮��ǡ�÷�Ӧ_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ������ѧ�ڻ�ͷ���ԣ����ۣ���ѧ���� ���ͣ������
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
|
ʵ���� |
HA��Ũ��/��mol/L�� |
NaOH��Ũ��/��mol/L�� |
�����Һ��pH |
|
�� |
0.2 |
0.2 |
pH=a |
|
�� |
c |
0.2 |
pH=7 |
|
�� |
0.2 |
0.1 |
pH��7 |
|
�� |
0.1 |
0.1 |
pH=9 |
��ش�
��1��������������ʵ���������Ӽ��������������a 7(�����������������)��HAΪ���
��2���������������ʵ���������������������������������һ���������
A.����Һ�����ʵ���Ũ�ȴ��ڼ���Һ
B.����Һ��H+��Ũ�ȴ��ڼ���Һ��OH����Ũ��
C.����Һ�����ʵ���Ũ��С�ڼ���Һ
D.����Һ��H+��Ũ��С�ڼ���Һ��OH����Ũ��
E.������Һ�����ʵ���Ũ�����
��3���ӱ���ʵ����������HA�� �ᣨ�ǿ������������ ����ʱ10mL0.5mol/LNaA��Һ��6mL1mol/L�����Ϻ���Һ�г�OH����ĸ�����Ũ���ɴ�С˳��Ϊ ��
��4������ʵ�����û����Һ����ˮ���������c(OH��)= mol/L��
��5������HA��һԪ���ᣬ��������CaA2��ˮ�д����ܽ�ƽ�⣺CaA2(s)  Ca2++2A-
Ca2++2A-
��H��0��һ���¶���CaA2������Һ��Ksp=c(Ca2+)c2(A2-)Ϊһ������
���¶�����ʱ��Ksp �����������С�����䡱����
�ڲ��25��ʱ��CaA2��KspΪ4.0��10-11�������½�10g CaA2����Ͷ��100mLCaCl2��Һ�У���ֽ�������й���ʣ�࣬�����Һ��c(Ca2+)=0.1mol/L������Һ��c(A2-)= ��
��ƽ�ⳣ�������˷����ϵ�Ŀ��淴Ӧ�ڸ������¶��½��еij̶ȣ�����ͬһ�����͵ķ�Ӧ��ƽ�ⳣ��Խ������Ӧ���еij̶�Խ��


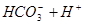









����������̼��ʹ�����ĵ���ƽ�ⳣ����д����������������������Ӧ�����ӷ���ʽ��
(1)������������ͨ�뵽������̼������Һ��___ _____________��
(2)������̼���ư���1�U1�����ʵ���֮��ǡ�÷�Ӧ_______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com