
 �����մɵ���������Al2O3���»�ԭ���Ʊ���Al2O3+3C+N
�����մɵ���������Al2O3���»�ԭ���Ʊ���Al2O3+3C+N
| ||
| ||
| m |
| 41n |
| 17m |
| 41n |
| 17m |


 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ��Դ�ص�һ��ѧ������һ���¿���ѧ�Ծ����������� ���ͣ������
��18�֣����������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�����ʡ�
������������ȡ����������������ͼ��ʾ��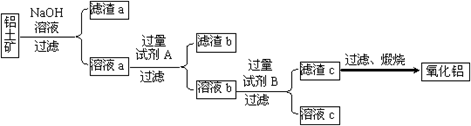
��1���Լ�A��������������������Һb���Լ�B��Ӧ�����ӷ���ʽΪ����������������
��2������NaOH��Һ���еķ�Ӧ�����ӷ���ʽΪ������������������ ___��
������������������������������������������������Һa��ͨ�������CO2�����õ��IJ��������պ�Ҳ�ɵõ�Al2O3���÷�����ȱ����������������������������
������ڵ����������Ʊ�������
��3��д�����Ļ�ѧ����ʽ������������������������������������ÿ����0.27������������ת�Ƶ��ӵ����ʵ���Ϊ����������������mol��
�������մɵ����������������ַ����Ʊ�
��4�������������»�ԭ������Al2O3 +��C +�� N2 ����AlN +��CO����ƽ��
����AlN +��CO����ƽ��
���Ȼ����백�����ºϳɷ���AlCl3+NH3 AlN+3HCl
AlN+3HCl
��5�������ڱȷ������������ϸ������ơ�����˵���У���ȷ������������������
A���������е� Al2O3��C��N2�ṹ�ȶ�����Ӧʱ�ƻ���ѧ����Ҫ���ĸ��������
B���������е�Al2O3��C���ײ����ڵ�������
C�����ַ����е�������Ϊ��ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ������һ���¿���ѧ�Ծ��������棩 ���ͣ������
��18�֣����������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�����ʡ�
������������ȡ����������������ͼ��ʾ��
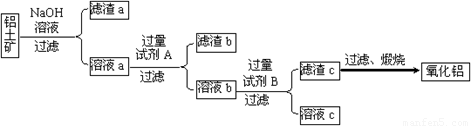
��1���Լ�A���������������� ����Һb���Լ�B��Ӧ�����ӷ���ʽΪ�������������� ��
��2������NaOH��Һ���еķ�Ӧ�����ӷ���ʽΪ������������������ ___��
������������������������������������������������Һa��ͨ�������CO2�����õ��IJ��������պ�Ҳ�ɵõ�Al2O3���÷�����ȱ�������������������������� ��
������ڵ����������Ʊ�������
��3��д�����Ļ�ѧ����ʽ���������������������������������� ��ÿ����0.27������������ת�Ƶ��ӵ����ʵ���Ϊ���������������� mol��
�������մɵ����������������ַ����Ʊ�
��4�������������»�ԭ������Al2O3 +��C +�� N2  ����AlN +��CO����ƽ��
����AlN +��CO����ƽ��
���Ȼ����백�����ºϳɷ���AlCl3+NH3  AlN+3HCl
AlN+3HCl
��5�������ڱȷ������������ϸ������ơ�����˵���У���ȷ���������������� ��
A���������е� Al2O3��C��N2�ṹ�ȶ�����Ӧʱ�ƻ���ѧ����Ҫ���ĸ��������
B���������е�Al2O3��C���ײ����ڵ�������
C�����ַ����е�������Ϊ��ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������Ҫ�ɷ���![]() ��������
��������![]() ��
��![]() �����ʡ�
�����ʡ�
������������ȡ����������������ͼ��ʾ��

��1���Լ�A�� ����Һb���Լ�B��Ӧ�����ӷ���ʽΪ ��
��2������Һa��ͨ�������CO2�����õ��IJ��������պ�Ҳ�ɵõ�Al2O3���÷�����ȱ���� ��
������ڵ����������Ʊ�������
��3�����ʱ�������ĵ缫��Ӧ����ʽΪ ��ÿ����0.324������������ת�Ƶ��ӵ����ʵ���Ϊ mol��
�������մɵ����������������ַ����Ʊ�
��4�������������»�ԭ���� ![]()
![]()
![]()
![]()
![]() CO������ƽ��
CO������ƽ��
���Ȼ����백�����ºϳɷ���![]()
![]()
![]()
��5�������ڱȷ������������ϸ������ơ�����˵���У���ȷ���� ��
A���������е� Al2O3��C��N2�ṹ�ȶ�����Ӧʱ�ƻ���ѧ����Ҫ���ĸ��������
B���������е�Al2O3��C���ײ����ڵ�������
C�����ַ����е�������Ϊ��ԭ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com