

| 5.62g+200g |
| 1000g?kg-1 |
| 5.62g+200g |
| 1000g?kg-1 |
CpKj?(kg?��)-1��
| ||
|
CpKj?(kg?��)-1��
| ||
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
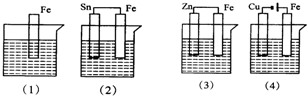 ��ͼ��������ʢ�к�ˮ���������и�ʴʱ�ɿ쵽����˳���ǣ�������
��ͼ��������ʢ�к�ˮ���������и�ʴʱ�ɿ쵽����˳���ǣ�������| A����4������2������1������3�� |
| B����2������1������3������4�� |
| C����4������2������3������1�� |
| D����3������2������4������1�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
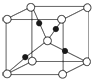 ����������һ�ֽྻ����������Դ��������������Ҫ�ɷ�ΪCO��CO2��H2�ȣ���H2��ϣ����ϳɼ״��������������õķ���֮һ��
����������һ�ֽྻ����������Դ��������������Ҫ�ɷ�ΪCO��CO2��H2�ȣ���H2��ϣ����ϳɼ״��������������õķ���֮һ��| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
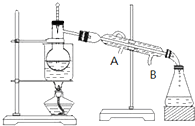
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
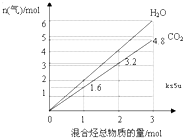 ������̬��A��B��ɵĻ��������ȫȼ�պ�õ�CO2��H2O��g�������ʵ����������������ʵ����ı仯��ͼ��ʾ����
������̬��A��B��ɵĻ��������ȫȼ�պ�õ�CO2��H2O��g�������ʵ����������������ʵ����ı仯��ͼ��ʾ����| ��ϱ�� | A�ķ���ʽ | B�ķ���ʽ | A��B�����ʵ����� |
| �� | CH4 | C2H4 | 2��3 |
| �� | CH4 | C3H4 | 7��3 |
| �� | CH4 | C4H4 | 4��1 |
| �� | |||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
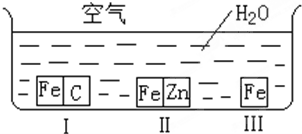
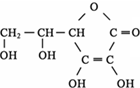 ��
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com