


 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ڵ�NaOH | B����˿ |
| C��ϡ���� | D��NaCl��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ��ƽ�й������뵰������Һ��Ӳ�����Կ���һ��������ͨ�� |
| B����������������ˮ |
| C����̬�����������ɢ�ڲ������Ƴ���ɫ���� |
| D�����Ȼ�����Һ�м���NaOH��Һ���ֺ��ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� | ���� | |
| A | �μ�AgNO3��Һ | ���ɰ�ɫ���� | ԭ��Һ����Cl- |
| B | �μ���ˮ��CCl4�������� | �²���Һ���Ϻ�ɫ | ԭ��Һ����I- |
| C | ��װ��Fe��NO3��2��Һ���Թ��м���ϡH2SO4 | �ڹܿڹ۲쵽����ɫ���� | HNO3�ֽ����NO2 |
| D | �μ�ϡNaOH��Һ����ʪ���ɫʯ����ֽ�����Թܿ� | ��ֽ������ | ԭ��Һ����NH4+ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
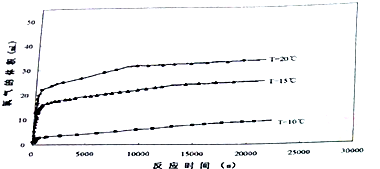 ������ɫ������N205�ϴ�ͳ���������з�Ӧ��ЧӦС���¶������ơ����������ŵ㣬����õ��㷺��Ӧ�ã�
������ɫ������N205�ϴ�ͳ���������з�Ӧ��ЧӦС���¶������ơ����������ŵ㣬����õ��㷺��Ӧ�ã�| t/s | 0 | 500 | 1000 | 1500 |
| c��N2O5��/mol��L-1 | 4.00 | 3.52 | 2.00 | 2.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | HCN | HF | CH3COOH | HNO2 |
| ���볣�� | 6.2��10-10 | 6.8��10-4 | 1.8��10-5 | 6.4��10-4 |
| A��HCN��HNO2��CH3COOH��HF |
| B��HF��HNO2��CH3COOH��HCN |
| C��HCN��CH3COOH��HNO2��HF |
| D��HCN��CH3COOH��HF��HNO2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com