
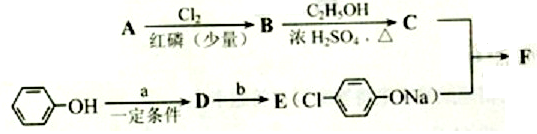
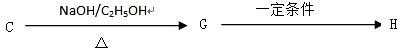
 G�ķ�Ӧ����Ϊ_____________________.
G�ķ�Ӧ����Ϊ_____________________.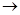 H�ķ�Ӧ����ʽ��_______________________��
H�ķ�Ӧ����ʽ��_______________________�� (CH3)2CClCOOC2H5+H2O��
(CH3)2CClCOOC2H5+H2O��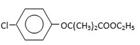
 (CH3)2CClCOOC2H5+H2O��
(CH3)2CClCOOC2H5+H2O��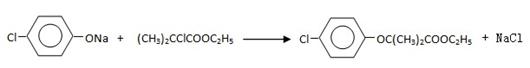 ����˸÷�Ӧ����ȡ����Ӧ��
����˸÷�Ӧ����ȡ����Ӧ��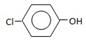 ������a��b���������Լ��ֱ���������NaOH��Һ��
������a��b���������Լ��ֱ���������NaOH��Һ��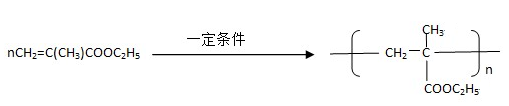 ��
��

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �������еİ����ױ�������
�������еİ����ױ������� ��
��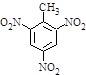 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
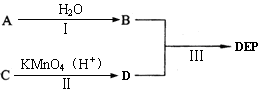
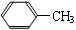
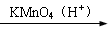
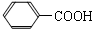
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
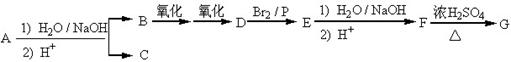
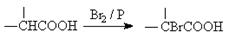
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
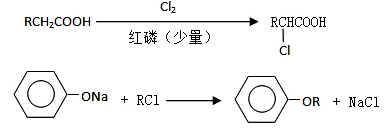
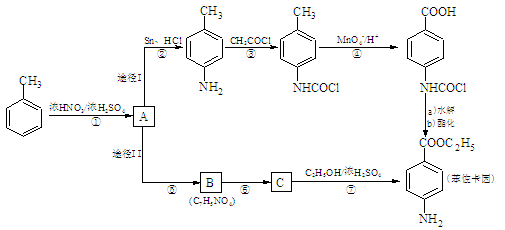
 2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��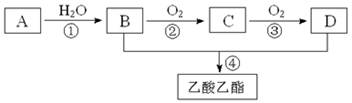
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
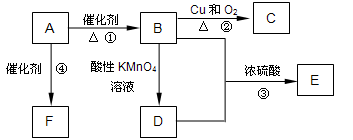
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
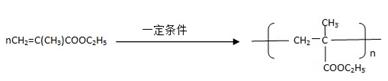
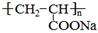 ����һ��ǿ��ˮ��֬����ij��A�ϳɾ۱�ϩ���Ƶ��������£�
����һ��ǿ��ˮ��֬����ij��A�ϳɾ۱�ϩ���Ƶ��������£�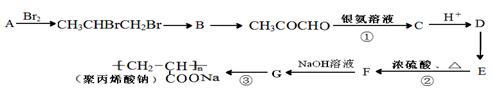
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
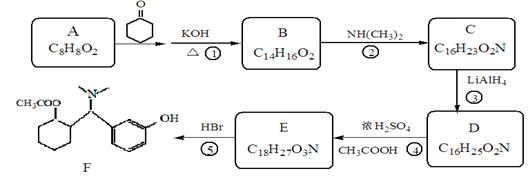
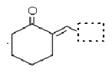
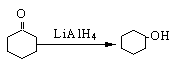
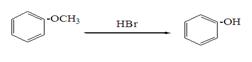
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
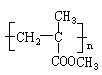 �ĵ������������ƹ��Ⱥ����ϵ�У�������������ܵõ������л�����еģ� �����������֮һ��Ϊͬ���칹�塣
�ĵ������������ƹ��Ⱥ����ϵ�У�������������ܵõ������л�����еģ� �����������֮һ��Ϊͬ���칹�塣| A������ | B����ϩ�� | C��������� | D�������ϩ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com