
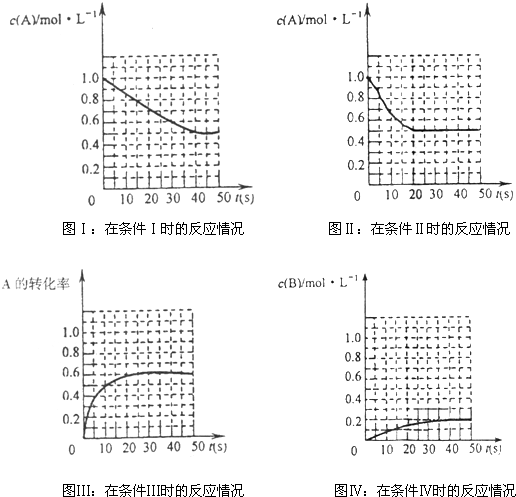
| A��������������ʱ�ķ�Ӧ�¶Ȳ�ͬ��ѹǿ��ͬ | B��������ʱ����δʹ�ô�����������ʱ����ʹ���˴��� | C��������ʱ��ƽ�������У�����C��Ũ�ȵ���0.6mol?L-1 | D������������������Ƚϣ�������ʱ���������˷�Ӧ��ϵ��ѹǿ |
| 1 |
| 2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | H2 | CO | |||
| 1 | 650 | 2 | 4 | 1.6 | 2.4 | 6 |
| 2 | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ʼŨ�� | �� | �� | �� |
| c��H2��/mol/L | 0.010 | 0.020 | 0.020 |
| c��CO2��/mol/L | 0.010 | 0.010 | 0.020 |
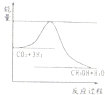
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ٵƾ���ʹ�������������ܻ������ŵ㣮һ���¶��£��ڵ��ٵƵ����ڷ���������������ڵ��ݱ��ϵ��ٿ��Է������µĿ��淴Ӧ��
���ٵƾ���ʹ�������������ܻ������ŵ㣮һ���¶��£��ڵ��ٵƵ����ڷ���������������ڵ��ݱ��ϵ��ٿ��Է������µĿ��淴Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ʱ�� \COת���� \�¶� |
1Сʱ | 2Сʱ | 3Сʱ | 4Сʱ |
| T1 | 30% | 50% | 80% | 80% |
| T2 | 35% | 60% | a1 | a2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и�����һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
�˹��̵���ָ����Ԫ��������̬ת��Ϊ����̬�Ĺ��̡�
I.���һЩ��ѧ���о����ø����ӵ����Ե�SCY�մɣ��ܴ���H+��ʵ�鵪�Ĺ̶�һ��ⷨ�ϳɰ����������˵�����������ת���ʡ��ܷ�ӦʽΪ��N2(g)��3H2(g)  2NH3(g)�����ڵ�ⷨ�ϳɰ��Ĺ����У�Ӧ��H2���ϵ�ͨ��_________����������������� ������һ�缫ͨ��N2���õ缫�ķ�ӦʽΪ__________________________��
2NH3(g)�����ڵ�ⷨ�ϳɰ��Ĺ����У�Ӧ��H2���ϵ�ͨ��_________����������������� ������һ�缫ͨ��N2���õ缫�ķ�ӦʽΪ__________________________��
II.�ݱ�������һ�������£�N2�ڲ��������������Ķ������Ѵ�����������ˮ������Ӧ����Ҫ����ΪNH3����Ӧ�ķ�Ӧ����ʽΪ��2N2(g)��6H2O(g) 4NH3(g)��3O2(g) ��H��Q��
4NH3(g)��3O2(g) ��H��Q��
��1��������Ӧ��ƽ�ⳣ������ʽΪ_______________��
��2��ȡ��ݵ����N2��H2O�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ���������ͬ�ĺ����ܱ������У����¶Ȳ���ͬ������·�����Ӧ����Ӧ��ͬʱ���õ������������ �뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��ʾ����������Ӧ��Q________0�����������������=������
�뷴Ӧ�¶�T�Ĺ�ϵ������ͼ��ʾ����������Ӧ��Q________0�����������������=������

��3����������Ӧ���д���������·���������ͼ��ʾ��a��b��c��d���������У��ܱ�ʾ��Ӧ��ϵ�����仯����_______��ѡ����ĸ���ţ���ͼ�С�H�ľ���ֵΪ1530kJ��mol-1��

III.Ŀǰ��ҵ�ϳɰ���ԭ���ǣ�N2(g)��3H2(g)  2NH3(g) ��H����93.0kJ/mol��
2NH3(g) ��H����93.0kJ/mol��
�ش��������⣺
��1�����II�е����ݣ���O2(g)��2H2(g)��2H2O(g)�ġ�H��______________��
��2����һ���¶��£���1molN2��3mol H2����������������ܱ������з�����Ӧ���ﵽƽ��״̬ʱ��������������ʵ���Ϊ2.8mol��
�ٴ�ƽ��ʱ��H2��ת���ʦ�1��______________��
������ͬ�����£�����ʼʱֻ��NH3���ڸ������У��ﵽƽ��״̬ʱNH3��ת����Ϊ��2������1����2��1ʱ������ʼʱNH3�����ʵ���n(NH3)��_____________mol��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com