
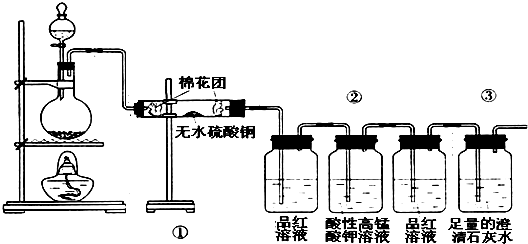
 2S02��+C02��+2H2O��SO2��ʹƷ����Һ��ɫ�Լ����Ը��������Һ��ɫ��ͨ�����Ը��������Һ��ȥSO2��CO2����ʹƷ����Һ��ɫ�Լ����Ը��������Һ��ɫ��
2S02��+C02��+2H2O��SO2��ʹƷ����Һ��ɫ�Լ����Ը��������Һ��ɫ��ͨ�����Ը��������Һ��ȥSO2��CO2����ʹƷ����Һ��ɫ�Լ����Ը��������Һ��ɫ�� 2S02��+C02��+2H2O
2S02��+C02��+2H2O

 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
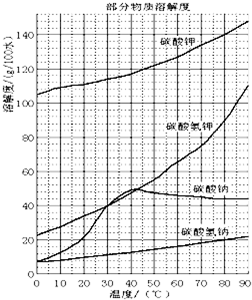
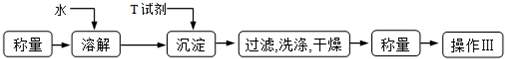
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | ��������� mL �� | ��NaOH��Һ�������mL�� |
| 1 | 20.00 | 18.20 |
| 2 | 17.10 | |
| 3 | 16.90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
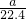 mol?L-1
mol?L-1 mol?L-1
mol?L-1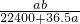 mol?L-1
mol?L-1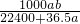 mol?L-1
mol?L-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
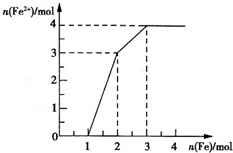 ����Fe3+��Cu2+��H+��NO
����Fe3+��Cu2+��H+��NO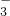 ��ijϡ��Һ������Fe�ۣ�Fe2+��Fe�۵����ʵ�����ϵ��ͼ��ʾ����ԭϡ��Һ��Fe3+��Cu2+��H+�����ʵ���֮��Ϊ
��ijϡ��Һ������Fe�ۣ�Fe2+��Fe�۵����ʵ�����ϵ��ͼ��ʾ����ԭϡ��Һ��Fe3+��Cu2+��H+�����ʵ���֮��Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
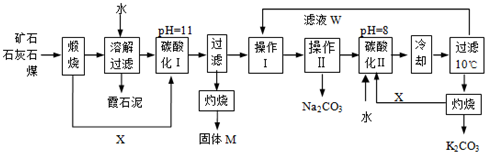
 ���жϳ����Ƿ�ϴ�Ӹɾ�������
���жϳ����Ƿ�ϴ�Ӹɾ�������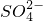 ������______
������______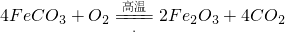 ��������464.0kg��FeCO3���õ�316.8kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������______kg��Ħ������/g?mol-1��FeCO3-116�� Fe2O3-160�� FeO-72��
��������464.0kg��FeCO3���õ�316.8kg��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������______kg��Ħ������/g?mol-1��FeCO3-116�� Fe2O3-160�� FeO-72���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com