
����Ŀ����������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)��ͼ1��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
CH3OH(g)+H2O(g)��ͼ1��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��

��1��д���÷�Ӧ���Ȼ�ѧ����ʽ__________________________��
��2�����ڸ÷�Ӧ������˵���У���ȷ����___________��
A����H>0����S>0 B����H>0����S<0
C����H<0����S<0 D����H<0����S>0
��3���÷�Ӧ��ƽ�ⳣ��K�ı���ʽΪ��________________��
��4���¶Ƚ��ͣ�ƽ�ⳣ��K____________������� �����䡱��С������
��5��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1 L���ܱ������У�����1 molCO2��3 molH2�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ2��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v (H2)=______________________��
��6�����д�ʩ����ʹn(CH3OH)/n(CO2)�������____________��
A�������¶� B���������
C����H2O(g)����ϵ�з��� D������He(g)��ʹ��ϵ��ѹǿ����
���𰸡�CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H������n-m��kJmol1Cc(CH3OH)��c(H2O)/c(CO2)��c(H2)3����0.225mol/��L.min��C
CH3OH(g)+H2O(g) ��H������n-m��kJmol1Cc(CH3OH)��c(H2O)/c(CO2)��c(H2)3����0.225mol/��L.min��C
��������������(1).�����Ȼ�ѧ����ʽ�ĺ��弰ͼ1�ش𣻣�2���������������С�ڷ�Ӧ���������Ϊ���ȷ�Ӧ���������ʵ���Խ����ֵԽС����3�������¶ȣ����ȷ�ӦK�����ȷ�ӦK��С����4������ƽ�ⳣ������ش𣻣�5����Ӧ����v=![]() ����6������ƽ���ƶ�ԭ�����
����6������ƽ���ƶ�ԭ�����
(1).�ʱ�������������������ȥ�������������������ͼʾ�÷�Ӧ���Ȼ�ѧ����ʽ CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H������n-m��kJmol1����2������ͼʾ�������������С�ڷ�Ӧ�����������������H<0���÷�Ӧ�������ʵ������٣������ؼ���Ӧ����S<0����3���÷�Ӧ��ƽ�ⳣ��K�ı���ʽΪc(CH3OH)��c(H2O)/c(CO2)��c(H2)3 ����4��CO2(g)+3H2(g)
CH3OH(g)+H2O(g) ��H������n-m��kJmol1����2������ͼʾ�������������С�ڷ�Ӧ�����������������H<0���÷�Ӧ�������ʵ������٣������ؼ���Ӧ����S<0����3���÷�Ӧ��ƽ�ⳣ��K�ı���ʽΪc(CH3OH)��c(H2O)/c(CO2)��c(H2)3 ����4��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)�Ƿ��ȷ�Ӧ���¶Ƚ��ͣ�ƽ�������ƶ���ƽ�ⳣ��K����5���ӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ������v��CO2��=
CH3OH(g)+H2O(g)�Ƿ��ȷ�Ӧ���¶Ƚ��ͣ�ƽ�������ƶ���ƽ�ⳣ��K����5���ӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ������v��CO2��=![]() =
=![]() =0.075mol/��Lmin�����������ʱȵ���ϵ���ȣ�v (H2)= 0.025mol/��L.min������6����H<0�������¶ȣ�ƽ�������ƶ���n(CH3OH)/n(CO2)��С��A���������ƽ�ⲻ�ƶ���n(CH3OH)/n(CO2)���䣬B����H2O(g)����ϵ�з��룬ƽ�������ƶ���n(CH3OH)/n(CO2)����C��ȷ�����ݳ���He(g)��ƽ�ⲻ�ƶ���n(CH3OH)/n(CO2)���䣬D����
=0.075mol/��Lmin�����������ʱȵ���ϵ���ȣ�v (H2)= 0.025mol/��L.min������6����H<0�������¶ȣ�ƽ�������ƶ���n(CH3OH)/n(CO2)��С��A���������ƽ�ⲻ�ƶ���n(CH3OH)/n(CO2)���䣬B����H2O(g)����ϵ�з��룬ƽ�������ƶ���n(CH3OH)/n(CO2)����C��ȷ�����ݳ���He(g)��ƽ�ⲻ�ƶ���n(CH3OH)/n(CO2)���䣬D����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е����λ����ͼ����Zԭ�ӵ������������ǵ�һ���������3��������˵������ȷ����

A. X�������̬�⻯���ˮ��Һ�Լ���
B. ����������Ӧˮ���������W��Zǿ
C. Z�ĵ�����������Ӧ��Y������������Ӧ����
D. X��ԭ�Ӱ뾶С��Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�Ŀǰ����������Ը�������Ҫ�ɷ�Ϊ FeO��Cr2O3��������Al2O3��MgO�����ʣ�Ϊ��Ҫԭ��������ˮ���ظ����ƣ�Na2Cr2O7��2H2O��������Ҫ������������ͼ��ʾ�� ���������漰����Ҫ��Ӧ�ǣ�
4FeO��Cr2O3+8Na2CO3+7O2=8Na2CrO4+2Fe2O3+8CO2��
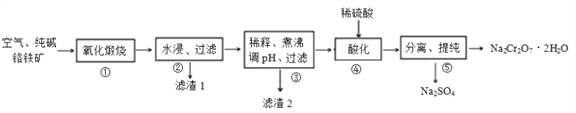
�ش��������⣺
��1����ˮ���ظ����ƣ�Na2Cr2O7��2H2O����Cr�Ļ��ϼ�Ϊ ______�� ��������ʱ������������Ŀ���� ____________��
��2���������� Al2O3 �봿�Ӧת��Ϊ�������Σ�д�� Al2O3�봿�Ӧ�Ļ�ѧ����ʽ________������1����Ҫ�ɷ�ΪMgO��_______�� �ѧʽ�� ��
��3������2�ijɷ���___________�� �ѧʽ�� ��
��4��������������Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��Ϊ��ɫ���õ�Na2Cr2O7�� Na2SO4�Ļ����Һ��������Ӧ�����ӷ���ʽΪ__________��
��5���ù��յ����Է�Һ�к���Cr2O72-�����Һ�м����̷���FeSO4��7H2O����ԭ��������Ӧ�����ӷ���ʽ��__________�� �����������Һ�м���ʯ��ˮ��ʹ c(Cr3+)����10-5mol/L�� ��ʱ��Һ��pHֵΪ ______(��֪���������£�Ksp[Cr(OH)3]=1.0��10-32)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬ�����͵�������������ijЩ��ͬ�����ʣ����������в��������ͨ�Ե��ǣ�������
A.��Ӧ�����κ�ˮ
B.ʹ��ɫʯ����Һ����ɫ
C.����ý�����Ӧ�����κ�����
D.�������Ʒ�Ӧ�����κ�ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����½ṹ�մ������������ȣ��ŵ���
A. ������ʴ����������
B. �ܶȴ�Ӳ�ȴ�
C. ���Ժã������������ʹ�����
D. ���õĴ��ȵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������һƿ����һ�������ʵ��ռ���Ʒ��ijѧ�����к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ����������������Ӧ�������ʵ��ش�

��1����ȷ��ȡ��5g�ռ���Ʒ���100 mL����Һ����Ҫ����Ҫ��������Ͳ���ձ���
��������������ƽ�⣬�������õ��������У�____________��_____________��
��2��ȡ10.00 mL����Һ��ѡ����ͼ��_________����A��B������ȡ��
��3����0.5000mol/L������ζ������ռ���Һ���Է�̪Ϊָʾ�����ζ�ʱ����
��ת�ζ��ܲ������������ֲ�ͣ��ҡ����ƿ������ע��___________________��
ֱ���ζ��յ㡣�ζ��ﵽ�յ�ı�־�ǣ�_______________________________��
(4)�����������ݣ��ռ�Ĵ���Ϊ��_______________________

��5���ж����в������������ƫ��ƫС����Ӱ�죩
�ٵζ�ǰ������ȷ���ζ��յ����ʱ����_________________
��װ����Һǰ����ƿ�ڲ�����������ˮ_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������Ŀռ乹����ͬ����
��NH3��H2O ��NH4+��H3O�� ��NH3��H3O�� ��O3��SO2
��CO2��BeCl2 ��SiO44����SO42�� ��BF3��Al2Cl6
A��ȫ�� B�����ܢޢ����� C���ۢܢݢ� D���ڢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯����������������Ӧ�ù㷺�� ��ת����ϵ����ͼ��ʾ��

��1��NH3�ĵ���ʽ��__________��
��2��ii �� NH3����ʱ�������·�Ӧ��
4NH3(g)��5O2(g) = 4NO(g)��6H2O(g) ��H1����907.28 kJ��mol-1
4NH3(g)��6NO(g)=5N2(g)��6H2O(g) ��H2����1811.63kJ��mol-1
��4NH3(g)��3O2(g)=2N2(g)��6H2O(g) ��H3��_________kJ��mol-1
��3��iv�з�Ӧ 2NO(g)+O2(g) ![]() 2NO2(g)�� ����������ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ��p1�� p2�������¶ȱ仯��������ͼһ��ʾ�� �� p1_____ p2 (�>����<�������� ������ͬ)��
2NO2(g)�� ����������ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ��p1�� p2�������¶ȱ仯��������ͼһ��ʾ�� �� p1_____ p2 (�>����<�������� ������ͬ)��
�÷�Ӧ����H ______0��A��B ����Ļ�ѧ��Ӧ���ʣ�v(A) ______v(B) �� ��֪ 400��ʱ�� Ͷ����NO��O2����ʼ���ֱ�Ϊx mol ��y mol����ʱ�������ΪV L��A ���ƽ�ⳣ�� K=______����x��y��V��ʾ�� ��
��4�����õ绯ѧ���ⷨ����ˮ�е���������Ⱦ����ͼ�����������м������ӽ���Ĥ��������ˮ����II���� ͨ��ʹNO3��ת��ΪN2��aΪ��Դ��______����Ag-Pt�缫�ϵĵ缫��Ӧʽ��________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������Ҵ�����������������ȼ�գ��õ�CO��CO2��ˮ��������Ϊ27.6�磬������ˮ������Ϊ10.8�磬��CO������Ϊ
A. 1.4�� B. 2.2�� C. 4.4�� D. 2.2~4.4��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com