
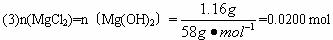
������������⣺
��1��A��ij�����Ļ�ѧʽΪ____________��������______________________��
��2��д��A�㵽B�㷢����Ӧ�����ӷ���ʽ_____________________________��
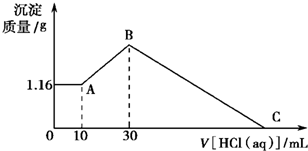
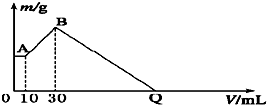
��3��ԭ�������MgCl2��������________g��AlCl3��������________g��NaOH��������________g��
��4��C��HCl��Һ�����Ϊ________mL��
��1��Mg(OH)2 ��Ϊ����10.0 mL HCl�����к���NaOH���ټ���HCl�������࣬˵��![]() ת��ΪAl(OH)3����A�����ΪMg(OH)2
ת��ΪAl(OH)3����A�����ΪMg(OH)2
(2)![]() +H++H2O====Al(OH)3��
+H++H2O====Al(OH)3��
(3)1.90 2.67 5.20 (4)130.0
��������1������10.0 mL HCl����������ӣ�˵������10.0 mL HCl���ðѹ�����NaOH�к͵�����A��ij�����ֻ��Mg(OH)2��
��2��AB��![]() �պ�����Al(OH)3����ӦΪ
�պ�����Al(OH)3����ӦΪ
![]() +H++H2O====Al(OH)3��
+H++H2O====Al(OH)3��
![]()
m(MgCl2)=0.0200 mol��95 g��mol-1=1.90 g
n(AlCl3)=n(![]() )=1.00 mol��L-1����30.0 mL-10.0 mL����10-3 L��mL-1=0.0200 mol
)=1.00 mol��L-1����30.0 mL-10.0 mL����10-3 L��mL-1=0.0200 mol
m(AlCl3)=0.0200 mol��133.5 g��mol-1=2.67 g
n(NaOH)=2n(MgCl2)+4n(AlCl3)+1.00 mol��L-1��0.0100 L=0.130 mol
m(NaOH)=0.130 mol��40.0 g��mol-1=5.20 g
(4)��B��C�������HCl�պð�Mg(OH)2��Al(OH)3�ܽ����
n��HCl��=30.0 mL��10-3 L��mL-1��1.00 mol��L-1+2n��Mg(OH)2��+3n��Al(OH)3��=0.130 mol
![]()


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ�����������õ�����Һ����μ���1mol?L-1 HCl��Һ������HCl��Һ�����V�����ɳ���������m�Ĺ�ϵ��ͼ��ʾ���Իش�?
��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ�����������õ�����Һ����μ���1mol?L-1 HCl��Һ������HCl��Һ�����V�����ɳ���������m�Ĺ�ϵ��ͼ��ʾ���Իش�?�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ����������������Һ����μ���1mol?L-1 HCl��Һ������HCl��Һ����������ɳ�����������ϵ��ͼ��ʾ���Իش�
��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ����������������Һ����μ���1mol?L-1 HCl��Һ������HCl��Һ����������ɳ�����������ϵ��ͼ��ʾ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣���NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ������������������Һ����μ���1.00mol/L HCl��Һ������HCl��Һ����������ɳ����Ĺ�ϵ��ͼ��ʾ��
�Իش�
��1��A��ij�����Ļ�ѧʽΪ ������ ��
��2��д��A�㵽B�㷢����Ӧ�����ӷ���ʽ ��
��3��ԭ�������MgCl2�������� ��AlCl3�������� ��NaOH�������� ��
��4��C����Һ�����Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ������һ���¿���ѧ�Ծ� ���ͣ������
��16�֣���NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ������������������Һ����μ���1.00mol/L HCl��Һ������HCl��Һ����������ɳ����Ĺ�ϵ��ͼ��ʾ��
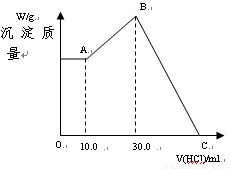
�Իش�
��1��A��ij�����Ļ�ѧʽΪ ������ ��
��2��д��A�㵽B�㷢����Ӧ�����ӷ���ʽ ��
��3��ԭ�������MgCl2�������� ��AlCl3�������� ��NaOH�������� ��
��4��C����Һ�����Ϊ mL��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com