
| A���٢ڢ� | B���ڢ� | C���٢ڢܢ� | D��ȫ�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
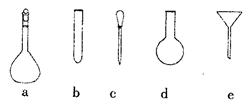
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
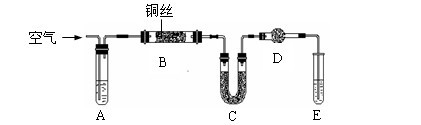
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
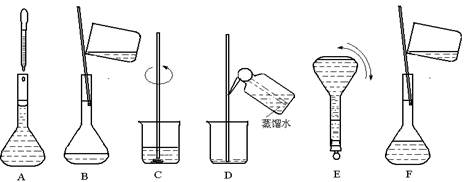
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥN2�е�����O2��ͨ�����ȵ�CuO��ĩ���ռ����� |
| B����ȥCO2�е�����HCl��ͨ��Na2CO3��Һ���ռ����� |
C����ȥNa Cl��Һ������CaCl2����������Na2CO3������ Cl��Һ������CaCl2����������Na2CO3������ |
| D����ȥKCl��Һ������MgCl2����������NaOH��Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 (�ƫ����ƫС������Ӱ�족)
(�ƫ����ƫС������Ӱ�족)| �ζ����� | ������Һ�����/mL | ����Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 1.02 | 20.99 |
| �ڶ��� | 25.00 | 2.00 | 22.03 |
| ������ | 25.00 | 0.00 | 22.00 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com