
��20�֣���֪����ȩ��һ�������¿���ͨ��Perkin��Ӧ��������ᣨ����45��50%������һ������AҲ�����ԣ���Ӧ����ʽ���£�
C6H5CHO������ȩ������CH3CO��2O �� C6H5CH��CHCOOH������ᣩ�� A
��1��������Ӧʽ�У���Ӧ������ʵ���֮��Ϊ1�U1������A�������� ��
��2��һ�������£���������Ҵ���Ӧ���������������������ṹ��ʽΪ______________��������˵����ȷ���� ��
a���ܷ����ӳɷ�Ӧ b����ʹ���Ը��������Һ��ɫ
c�������Ի���������¿���ˮ�� d�����ڷ�������������
��3��ȡ������ȩҲ�ܷ���Perkin��Ӧ����Ӧ����IJ������£�
|
ȡ������ȩ |
|
|
|
|
|
���ʣ�%�� |
71 |
63 |
52 |
82 |
�ɼ���ȡ������Perkin��Ӧ��Ӱ���У�д��2�����ɣ���[��Դ:ZXXK]
��____________________________________��__________________________________��
��4���屽��C6H5Br�����ϩ��������CH2��CHCOOC2H5���ڵ����ٴ��¿�ֱ�Ӻϳ�������������÷�Ӧ����Heck��Ӧ���ǻ�B��һ��ȡ����Ӧ���䷴Ӧ����ʽΪ
����Ҫ������Ӧ������________________________________________________
��5��Heck��Ӧ�У�Ϊ�˴ٽ���Ӧ�Ľ��У�ͨ���ɼ���һ���� ������ĸ�������ʣ�ԭ����__________________________________________________________________��
A�������� B�������� C������ D��ǿ����
��1�����ᣨ2�֣� ��2��C6H5CH��CHCOOC2H5��2�֣���abcd��2�֣�
��3������ԭ����ȩ��ԽԶ���Է�ӦԽ����������ԭ��ȡ��ʱ����λ����������λ�������3�֣� �ڱ�������ԭ��Խ�࣬�Է�ӦԽ������3�֣�zxxk
��4��C6H5Br��CH2��CHCOOC2H5��C6H5CH��CHCOOC2H5��HBr��3�֣�
��5��B��2�֣� �����к�HBr�������˷�Ӧ���Ũ�ȣ��ܴ�ʹƽ�������ƶ���3�֣�
����������1������ԭ���غ��֪��A�Ľṹ��ʽΪCH3COOH����A�����ᡣ
��2��������к����Ȼ����ܺ����Ҵ�����������Ӧ��������Ľṹ��ʽΪC6H5CH��CHCOOC2H5�����ڷ����к��б�����̼̼˫��������������ѡ��ȫ������ȷ�ģ�����ѡabcd��
��3������������IJ��ʿ��жϣ�����ԭ����ȩ��ԽԶ���Է�ӦԽ����������ԭ��ȡ��ʱ����λ����������λ������� �ڱ�������ԭ��Խ�࣬�Է�ӦԽ������
��4������ԭ���غ��֪����һ��������Ӧ����HBr�����Է���ʽΪC6H5Br��CH2��CHCOOC2H5��C6H5CH��CHCOOC2H5��HBr��
��5����Ϊ��Heck��Ӧ�����廯�����ɣ��������к�HBr�������˷�Ӧ���Ũ�ȣ��ܴ�ʹƽ�������ƶ������Դ�ѡB��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��07��㶫������10�֣���֪����ȩ��һ�������¿���ͨ��Perkin��Ӧ��������ᣨ����45��50%������һ������AҲ�����ԣ���Ӧ����ʽ���£�
C6H5CHO��(CH3CO)2O �� C6H5CH��CHCOOH�� A
����ȩ �����
��1��Perkin��Ӧ�ϳ������ķ�Ӧʽ�У���Ӧ������ʵ���֮��Ϊ11������A��������
��2��һ�������£���������Ҵ���Ӧ��������������������䷴Ӧ����ʽΪ________����Ҫ������Ӧ������
��3��ȡ������ȩҲ�ܷ���Perkin��Ӧ����Ӧ����IJ������£�
ȡ������ȩ |
|
|
|
|
���ʣ�%�� | 15 | 23 | 33 | 0 |
ȡ������ȩ |
|
|
|
|
���ʣ�%�� | 71 | 63 | 52 | 82 |
�ɼ���ȡ������Perkin��Ӧ��Ӱ���У�д��3�����ɣ���
��
��
��
��4���屽��C6H5Br�����ϩ��������CH2��CHCOOC2H5�����Ȼ��ٴ��¿�ֱ�Ӻϳ�������������÷�Ӧ����Beck��Ӧ���Ƿ��㻷�ϵ�һ��ȡ����Ӧ���䷴Ӧ����ʽΪ_________����Ҫ������Ӧ������
��5��Beck��Ӧ�У�Ϊ�˴ٽ���Ӧ�Ľ��У�ͨ���ɼ���һ���� ������ĸ��������
A ������ B ������ C ���� D ǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л���ѧ������(ѡ����)
28.��֪����ȩ��һ�������¿���ͨ��Perkin��Ӧ��������ᣨ����45��50%������һ������AҲ�����ԣ���Ӧ����ʽ���£�C6H5CHO+(CH3CO)2O �� C6H5CH=CHCOOH+ A
����ȩ �����
��1��Perkin��Ӧ�ϳ������ķ�Ӧʽ�У���Ӧ������ʵ���֮��Ϊ1�U1������A�������� ��
��2��һ�������£���������Ҵ���Ӧ��������������������䷴Ӧ����ʽΪ
����Ҫ������Ӧ������
��3��ȡ������ȩҲ�ܷ���Perkin��Ӧ����Ӧ����IJ������£�
ȡ������ȩ |
|
|
|
|
���ʣ�%�� | 15 | 23 | 33 | 0 |
ȡ������ȩ |
|
|
|
|
���ʣ�%�� | 71 | 63 | 52 | 82 |
�ɼ���ȡ������Perkin��Ӧ��Ӱ���У�д��3�����ɣ���
��
��
��
��4���屽��C6H5Br�����ϩ��������CH2=CHCOOC2H5�����Ȼ��ٴ��¿�ֱ�Ӻϳ�������������÷�Ӧ����Beck��Ӧ���Ƿ��㻷�ϵ�һ��ȡ����Ӧ���䷴Ӧ����ʽΪ
����Ҫ������Ӧ������
��5��Heck��Ӧ�У�Ϊ�˴ٽ���Ӧ�Ľ��У�ͨ���ɼ���һ���� ������ĸ��������
A ������ B ������ C ���� D ǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����ȩ��һ�������¿���ͨ��Perkin��Ӧ��������ᣨ����45��50%������һ������AҲ�����ԣ���Ӧ����ʽ���£�
C6H5CHO��(CH3CO)2O �� C6H5CH��CHCOOH�� A
����ȩ �����
��1��Perkin��Ӧ�ϳ������ķ�Ӧʽ�У���Ӧ������ʵ���֮��Ϊ1�U1������A�������� ��
��2��һ�������£���������Ҵ���Ӧ��������������������䷴Ӧ����ʽΪ
����Ҫ������Ӧ������
��3��ȡ������ȩҲ�ܷ���Perkin��Ӧ����Ӧ����IJ������£�
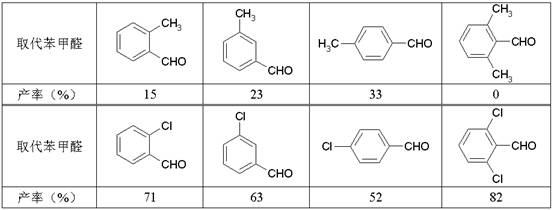
�ɼ���ȡ������Perkin��Ӧ��Ӱ���У�д��3�����ɣ���
��
��
��
��4���屽��C6H5Br�����ϩ��������CH2��CHCOOC2H5�����Ȼ��ٴ��¿�ֱ�Ӻϳ�������������÷�Ӧ����Beck��Ӧ���Ƿ��㻷�ϵ�һ��ȡ����Ӧ���䷴Ӧ����ʽΪ
����Ҫ������Ӧ������
��5��Beck��Ӧ�У�Ϊ�˴ٽ���Ӧ�Ľ��У�ͨ���ɼ���һ���� ������ĸ��������
A ������ B ������ C ���� D ǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��)���ݡ��й���ҩ��������������F�������Ʊ����ǵ䡱ҩƷ����������ʹ�����м�����ϳ�·��Ϊ��

![]() ��֪�� һ����������RNH2+
��֪�� һ����������RNH2+CH2Cl ��RNHCH2
+HCl��R��
������������
������ͬϵ���ܱ����Ը��������Һ�������磺
�����������������ԣ���������
��������Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ����
�ش��������⣺
��1��C�Ľṹ��ʽ�� ��
��2����д��D+E��F�Ļ�ѧ����ʽ�� ��
��3��E��һ�������£��ɾۺϳɺܺõĹ��ܸ߷��Ӳ��ϣ�д���ϳɴ˸߾���Ļ�ѧ����ʽ ��
��4����Ӧ��~���У�����ȡ����Ӧ���ǣ��Ӧ��ţ�
(5)��������������E��ͬ���칹�����Ŀ�ǣ� ����
����FeCl3��Һ����ɫ��Ӧ ���ܷ���������Ӧ �۱����ϵ�һ��ȡ����ֻ��3�֡�
A��3�� B��8�� C��10�� D��12��
(6) ��֪����ȩ��һ�������¿���ͨ��Perkin��Ӧ��������ᣨ����45~50%������Ӧ����ʽ���£�
C6H5CHO+ (CH3CO)2O �� C6H5CH=CHCOOH+CH3COOH
����ȩ �����
������ȩ�ı�������ȡ������Ҳ�ܷ���Perkin��Ӧ����Ӧ����IJ������£�
|
��Ӧ��
|
|
|
|
|
| ���ʣ�%�� | 15 | 23 | 33 | 0 |
|
��Ӧ��
|
|
|
|
|
| ���ʣ�%�� | 71 | 63 | 52 | 82 |
������ϱ��ش�ȡ������Perkin��Ӧ��Ӱ���У�д��3�����ɣ���
��
��
�� [��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com