
ʱ�к���Ϊ57.3 kJ��mol-1���������Ȼ�ѧ����ʽ��д��ȷ����( )
��C8H18(1)+![]() O2(g)====8CO2(g)+9H2O(1)����H=+5 518.kJ��mol-1
O2(g)====8CO2(g)+9H2O(1)����H=+5 518.kJ��mol-1
��C8H18(1)+![]() O2(g)====8CO2(g)+9H2O(1)����H=-5 518kJ��mol-1
O2(g)====8CO2(g)+9H2O(1)����H=-5 518kJ��mol-1
��H++OH-====H2O����H=-57.3 kJ��mol-1
��NaOH(aq)+![]() H2SO4(aq)====
H2SO4(aq)====![]() Na2SO4(aq)+H2O(1)����H=+57.3kJ��mol-1
Na2SO4(aq)+H2O(1)����H=+57.3kJ��mol-1
A.�٢� B.�ڢ� C.�ڢ� D.ֻ�Т�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ͬ��ͬѹ�£�H2��g��+Cl2��g���T2HCl��g���ڹ��պ͵�ȼ�����µġ�H��ͬ | B��Ǧ���طŵ�ʱ�ĸ����ͳ��ʱ��������������ԭ��Ӧ | C����֪��H2��g��+I2��g��?2HI��g������H=-9.48 kJ/mol������254g I2��g����2gH2��g����ַ�Ӧ�ɷų�9.48 kJ������ | D����֪��101 kPaʱ��2 g̼ȼ������CO�ų�����ΪQ kJ����̼��ȼ����Ϊ6Q kJ?mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��(N2H4)�ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϡ���֪��101 kPaʱ��32.0 g N2H4����������ȫȼ�����ɵ������ų�����624 kJ(25��ʱ)��N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��____________________________________________________________________��
(2)��-����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������20%��30%��KOH��Һ��д����-����ȼ�ϵ�طŵ�ʱ���������ĵ缫��Ӧʽ��
������________________________________��
������________________________________
(3)ͼ2-2-5��һ���绯ѧ����ʾ��ͼ��
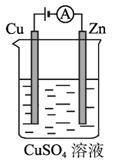
ͼ2-2-5
��пƬ�Ϸ����ĵ缫��Ӧ��________________________________________________��
�ڼ���ʹ����?����ȼ�ϵ����Ϊ�������еĵ�Դ��ͭƬ�������仯128 g������-����ȼ�ϵ�����������ı��״���µĿ���___________L(��������������������Ϊ20%)
(4)��ͳ�Ʊ��µķ���������NaClO����NH3���Ƶ��µ�ϡ��Һ���÷�Ӧ�����ӷ���ʽ��________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭�ٺ��а��Ƹ��и�һ��ѧ����ĩģ�⻯ѧ�Ծ����������� ���ͣ������
��6�֣�����֪��101 kPaʱ��CH4��ȫȼ������1molҺ̬ˮ���ų�������ΪQkJ����CH4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
����ͭƬ��пƬ��400 mLϡ������ɵ�ԭ����У�����·��ͨ��0.2 mol���ӣ�H2SO4ǡ�÷�Ӧ��ϡ��Լ��㣺
��1�����������������ڱ�״���£���
��2��ԭ400 mLϡ��������ʵ���Ũ�ȣ���������Һ������仯����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ�߶�3��������ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪����101 kPaʱ��2C(s)��O2(g)===2CO(g)����H����221 kJ/mol
��ϡ��Һ�У�H��(aq)��OH��(aq)===H2O(l)����H����57.3 kJ/mol���н�����ȷ����(����)
A��̼��ȼ���ȴ���110.5 kJ/mol
B���ٵķ�Ӧ��Ϊ 221 kJ/mol
C��ϡ������ϡNaOH��Һ��Ӧ���к���Ϊ��57.3 kJ/mol
D��Ũ������ϡNaOH��Һ��Ӧ����1 molˮ���ų�57.3 kJ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�������
��11�֣�CO��CH4��Ϊ�����Ŀ�ȼ�����塣
(1)�������CO��CH4����ͬ�����·ֱ���ȫȼ�գ�ת�Ƶĵ�����֮���� ��
(2)��֪��101 kPaʱ��CO��ȼ����Ϊ283 kJ/mol����ͬ�����£���2 molCH4��ȫȼ������Һ̬ˮ�����ų�������Ϊ1 mol CO��ȫȼ�շų�������6.30 ����CH4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
(3)120�桢101 kPa�£�a mL��CO��CH4��ɵĻ��������bmL O2����ȫȼ�պָ���ԭ�¶Ⱥ�ѹǿ��
�� �����������O2ǡ����ȫ��Ӧ������b mL CO2������������CH4���������Ϊ
������2λС������
�� ��ȼ�պ����������С��a/4 mL ����a��b��ϵ����ѧ��ʾʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com