��ѧ�ϰѺ��н϶�Ca2+��Mg2+��ˮ��ΪӲˮ���ҹ��涨����ˮ��Ӳ�Ȳ��ܳ���25�ȣ�Ӳ�ȵı�ʾ�����ǣ���ˮ�е�Ca2+��Mg2+������Ca2+�������������CaO��������ͨ����1Lˮ�к���10 mgCaO��Ϊ1�ȡ�
��ʹӲˮ������Ca2+��Mg2+��HCO3���ȳ�ȥ�ķ����ǣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ���ڳ����������ó�������Ҫ�ɷ�Ϊ_____________��___________����ѧʽ����
��FeSO4��7H2O�dz��õ����ۼ�������ˮ���������ɽ�״Fe(OH)3���������ۼ���ȥ�������������ˮ�����õĹ���______________����д��ţ���
�ٷ���ˮ�� �ڷ���������ԭ��Ӧ �۽���ľ۳�
�Ǿ����������ˮҪ��һ�����������õ���������Cl2��Ӧ��Cl2������ʵ����Cl2��ˮ��Ӧ������__________���ѧʽ����Cl2��ˮ��Ӧ�����ӷ���ʽΪ��_______________������Cl2��ˮ�е��л������ò��������岻�������ʣ���������������֯Ҫ��ֹͣ��Cl2������ˮ�������������������Ĵ���Ʒ���ҹ��Ѿ���ʼʹ��ClO2����Cl2������ˮ����������ClO2��Cl2������Ч�ʱ�Ϊ____________����λ����ת�Ƶ�����֮�ȣ�����������ԭ��Ӧ�Ƕȿ������������У�___________������Ϊ����Cl2�Ĵ���Ʒ����д��ţ���
��Ca(ClO)2 ��NH2Cl(�Ȱ�) ��K2FeO4 ��SO2 ��Al2(SO4)3
��ȡijˮ��10mL����ʵ��ⶨ�����к�Ca2+0.001g����Mg2+0.00048g����ͨ������ش𣬸�ˮ����Ӳ�ȽǶȿ����Ƿ��������ˮ������д��������̣�

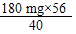 = 252 mg����ˮ����Ӳ��Ϊ252 mg/10 mL=25.2 �ȣ�25 �ȣ�����������ˮ����
= 252 mg����ˮ����Ӳ��Ϊ252 mg/10 mL=25.2 �ȣ�25 �ȣ�����������ˮ����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�