
 ���Ṥҵβ���ж�������������0.05%�����������ʱ�辭����������ŷţ�ijУ��ȤС�����ⶨ���Ṥҵβ���ж������������������·�����
���Ṥҵβ���ж�������������0.05%�����������ʱ�辭����������ŷţ�ijУ��ȤС�����ⶨ���Ṥҵβ���ж������������������·�����
| 22.4m |
| 233V |
| 22.4m |
| 233V |
| mg |
| 233g/mol |
| m |
| 233 |
| m |
| 233 |
| 22.4m |
| 233 |
| ||
| VL |
| 22.4m |
| 233V |
| 22.4m |
| 233V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O
Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O
Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O Mn3O4+SO2��+2SO3��+3H2O
Mn3O4+SO2��+2SO3��+3H2O Mn3O4+SO2��+2SO3��+3H2O
Mn3O4+SO2��+2SO3��+3H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| n(SO32-) |
| n(HSO3-) |
|
91��9 | 1��1 | 9��91 | ||
| ������pH | 8.2 | 7.2 | 6.2 |
| n(SO32-) |
| n(HSO3-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��(V)���仯�����ڹ�ҵ�����²��Ϻ�����Դ���������й㷺��Ӧ�ã����нӴ��������Ṥҵ�о�Ҫ�õ�V2O5��������
2SO2(g)��O2(g) 2SO3(g)����H��0��
ij�¶��£���2mol SO2��1mol O2����10 L�ܱ������У���V2O5�������¾�5min��Ӧ��ƽ�⣬SO2��ƽ��ת����(��)Ϊ80%��
��1��5min�� v(SO3 )��������������mol��L��1��min��1��
��2�����¶���ƽ�ⳣ��K����������������������
��3������С������������ﵽ�µ�ƽ�⣬��ͼ�л�����Ӧ���ʱ仯ͼ��
��4�����Ṥҵβ��SO2��Ũ��ˮ���գ���Ӧ�����ӷ���ʽ�������������������������������պ�IJ������տ��Ƴɷ�����泥ۼ�(NH4)2SO4�ݡ�
��5��ij���������P����ĵ�������ò�ͬ��̬���ӶԵ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã���ԭ������ͼ��ʾ��
���øõ�ص��(NH4)2SO4��Һ����(NH4)2S2O8(���������)�����ʱ���ö��Ե缫�������缫��Ӧʽ�ɱ�ʾΪ����������������������������������������1mol(NH4)2S2O8�����������H����������(������١�)��������mol��
�ڵ��ʹ��һ��ʱ��������г�磬�������У����缫��ӦʽΪ������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ���ݶ��и�����ѧ�ڵ�һ���ۺ���ϰ�����ۺ��Ծ�����ѧ���֣� ���ͣ������
��(V)���仯�����ڹ�ҵ�����²��Ϻ�����Դ���������й㷺��Ӧ�ã����нӴ��������Ṥҵ�о�Ҫ�õ�V2O5��������
2SO2(g)��O2(g)  2SO3(g)����H��0��
2SO3(g)����H��0��
ij�¶��£���2 mol SO2��1 mol O2����10 L�ܱ������У���V2O5�������¾�5min��Ӧ��ƽ�⣬SO2��ƽ��ת����(��)Ϊ80%��
��1��5min�� v(SO3 )��������������mol��L��1��min��1��
��2�����¶���ƽ�ⳣ��K����������������������
��3������С������������ﵽ�µ�ƽ�⣬��ͼ�л�����Ӧ���ʱ仯ͼ��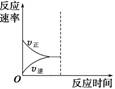
��4�����Ṥҵβ��SO2��Ũ��ˮ���գ���Ӧ�����ӷ���ʽ�������������������������������պ�IJ������տ��Ƴɷ�����泥ۼ�(NH4)2SO4�ݡ�
��5��ij���������P����ĵ�������ò�ͬ��̬���ӶԵ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã���ԭ������ͼ��ʾ��
���øõ�ص��(NH4)2SO4��Һ����(NH4)2S2O8(���������)�����ʱ���ö��Ե缫�������缫��Ӧʽ�ɱ�ʾΪ����������������������������������������1mol(NH4)2S2O8�����������H����������(������١�)��������mol��
�ڵ��ʹ��һ��ʱ��������г�磬�������У����缫��ӦʽΪ������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ϻ��������������ѧ����ĩ��һģ�����Ի�ѧ�Ծ��������棩 ���ͣ������
��Ԫ�صĺ��������ڹ�ҵ����;�㷺�����������ա�
��ҵ����Na2SO3��Һ������ҵβ���е�SO2���±����ݱ�ʾ��Ӧ������ ��pH�仯�Ĺ�ϵ��
��pH�仯�Ĺ�ϵ��
|
|
91:9 |
1:1 |
9:91 |
|
������pH |
8.2 |
7.2 |
6.2 |
��1������ = 1ʱ����ҺpH= 7.2��ԭ��___________________������0.20 mol/L ��NaOH��Һ����Ӧǰ����Һ������䣩����SO2������Ӧ����Һ�����ԣ���
= 1ʱ����ҺpH= 7.2��ԭ��___________________������0.20 mol/L ��NaOH��Һ����Ӧǰ����Һ������䣩����SO2������Ӧ����Һ�����ԣ���
c (HSO3-) + 2c (SO32-) = _______ mol/L ��
��2����֪��Ki1(H2SO3)> Ki(HAc) > Ki2(H2SO3) > Ki2(H2CO3)��ҪʹNaHSO3��Һ��c(Na+):c(HSO3-)�ӽ�1:1��������Һ�м�������____________��
a��H2SO3��Һ b��NaOH��Һ c�������� d��Na2CO3
��3��ʵ����ͨ�����µ��KHSO4��Һ�Ʊ����������K2S2O8��д������KHSO4�ĵ��뷽��ʽ��__________________________________________��
��4��S2O82-��ǿ�����ԣ���ԭ����ΪSO42-�������̣�MnSO4��������أ�K2S2O8����������Һ�������Ӵ��¿ɷ�����Ӧ���õ��Ϻ�ɫ��Һ����д�˷�Ӧ�Ļ�ѧ����ʽ�� ��
��5����֪��S2O32-�н�ǿ�Ļ�ԭ�ԣ�ʵ���ҿ���I-�ⶨK2S2O8��Ʒ�Ĵ��ȣ���Ӧ����ʽΪ��
S2O82-��2I-��2SO42-��I2 ������ I2��2S2O32-��2I-��S4O62- ������
S2O82-��S4O62-��I2������ǿ��˳��__________________________��
��6��K2S2O8��ƫ����ϩ��CH2=CF2���ۺϵ���������ƫ����ϩ��CH3��CClF2������ȥHCl�Ƶã�����0.5 molƫ����ϩ����Ҫ����54 kJ���ȣ�д����Ӧ���Ȼ�ѧ����ʽ_______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com