
��12�֣���ҵ�Ͽ�����ʯ����ԭ�������Ҵ�����Ӧ����ͼ��ʾ��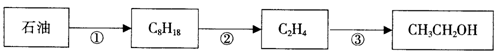
��1����ʯ���з�������͡����͡�ú�͵ķ����ǣ�
��2����Ӧ�۵Ļ�ѧ����ʽ ��
��3�����������в�������ˮ������Ӧ����
����ϩ���������NaOH��Һ��AgNO3��Һ���ѻ����͢����FeCl2��Һ
| A���ۢܢ� | B���٢� | C���� | D���ڢ� |

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ��
��1��������ֻ����̼��������Ԫ�أ��ڱ�״����Ϊ��̬���л���IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ҵ�Ͽ����ñ����������������ʣ���Ӧʽ��ʾ��| 500�� |
| Cu��Ag |
| �� |
| Cu��Ag |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ����һ�С�������ѧ2011��2012ѧ���һ��ѧ�ڵڶ���������ѧ���� ���ͣ�022
| |||||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012��㶫���ݡ�������ѧ��һ��ѧ�ڵڶ���������ѧ�Ծ��������棩 ���ͣ������
��12�֣���ҵ�Ͽ�����ʯ����ԭ�������Ҵ�����Ӧ����ͼ��ʾ��

��1����ʯ���з�������͡����͡�ú�͵ķ����ǣ�
��2����Ӧ�۵Ļ�ѧ����ʽ ��
��3�����������в�������ˮ������Ӧ����
�� ��ϩ�������� ��NaOH��Һ ��AgNO3��Һ ���ѻ����� ���� ��FeCl2��Һ
A���ۢܢ� B���٢� C���� D���ڢ�
��4�� ������������

��Ϊͬϵ����� ������ţ���ͬ������Ϊͬ���칹����� �� ��֪15g������ȫȼ������Һ̬ˮ�ų�akJ����������д��������ȫȼ�յ��Ȼ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������

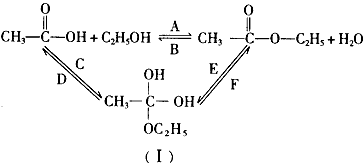
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com