
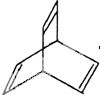
 I���л���Ľṹ���á�����ʽ����ʾ��CH3-CH=CH-CH3�ɼ�д
I���л���Ľṹ���á�����ʽ����ʾ��CH3-CH=CH-CH3�ɼ�д Ϊ���л���X�ļ���ʽ��ͼ��
Ϊ���л���X�ļ���ʽ��ͼ��| 300��4.91% |
| 14 |
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��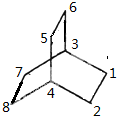 ��3��4λ��Hԭ����ͬ������λ��Hԭ����ͬ�����ж���ȡ����ȡ��λ���У�1��λ�ã�1��2��λ�ã�1��3��λ�ã�1��4��λ�ã�3��4��λ�ã�1��6��λ�ã�1��5��λ�ã�����λ���ظ����ʹ���7�֣��ʴ�Ϊ��7��
��3��4λ��Hԭ����ͬ������λ��Hԭ����ͬ�����ж���ȡ����ȡ��λ���У�1��λ�ã�1��2��λ�ã�1��3��λ�ã�1��4��λ�ã�3��4��λ�ã�1��6��λ�ã�1��5��λ�ã�����λ���ظ����ʹ���7�֣��ʴ�Ϊ��7��| 300��4.91% |
| 14 |
| 14 |
| 4.91% |
| 285��71.58% |
| 12 |
| 285��6.67% |
| 1 |
| 285��16.84% |
| 16 |


 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������£�N2H4���ǵ������ֳ���������ڿ�ѧ����������������Ҫ��Ӧ�ã�
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������£�N2H4���ǵ������ֳ���������ڿ�ѧ����������������Ҫ��Ӧ�ã�| �ݡ��� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 3mol��H2��2mol��N2 | 6mol��H2��4mol��N2 | 2mol��NH3 |
| �ﵽƽ���ʱ�䣨min�� | 5 | 8 | |
| N2��Ũ�ȣ�mol?L�� | c1 | 1.5 | |
| NH��������� | ��1 | ��2 | ��3 |
| ��������ܶȣ�g?L�� | ��1 | ��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������绰119��Ѱ��֧Ԯ |
| B���������źʹ����ر�ú��Դ |
| C��������ƣ�Ѱ��й©�� |
| D���������������̻��ų�ú�����ر�ú��Դ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com