
��13�֣���ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯���������Һ����ת����ϵ����֪B��C��D��E�Ƿǽ������ʣ����ڳ��³�ѹ�¶������壬������G����ɫ��ӦΪ��ɫ��������I��Jͨ��״���³���̬����Ӧ���ǻ��������е�һ����Ҫ�̵���Ӧ��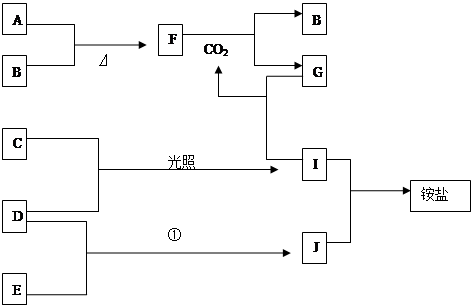
��ش��������⣺
��1��д���������ʻ�ѧʽ��A B C
(2)д���������ʼ䷴Ӧ�Ļ�ѧ����ʽ
A+B
F+CO2
D+E
I+J
(3)д�����з�Ӧ�����ӷ���ʽ
G+I

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯���������Һ����ת����ϵ����֪B��C��D��E�Ƿǽ������ʣ����ڳ����¶������壻������G����ɫ��ӦΪ��ɫ��������I��Jͨ��״���³���̬����Ӧ���ǻ��������е�һ����Ҫ�̵���Ӧ����ش��������⣺
��ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯���������Һ����ת����ϵ����֪B��C��D��E�Ƿǽ������ʣ����ڳ����¶������壻������G����ɫ��ӦΪ��ɫ��������I��Jͨ��״���³���̬����Ӧ���ǻ��������е�һ����Ҫ�̵���Ӧ����ش��������⣺

| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
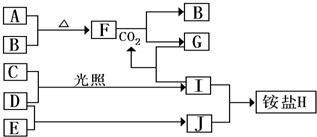
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�밴Ҫ������������⣺
��1����Ӧ�۵Ļ�ѧ����ʽ__________________________________________________��
��2��C�Ľṹʽ_______________��H�Ļ�ѧʽ_____________________��
��3��L����Һ�뻯����E��Ӧ�����ӷ���ʽ_______________________________________��
��4��������J�Ļ�ѧʽ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ̩���и����ڶ��ָ�ϰ����������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ�Dz��ֶ�����Ԫ�ػ��ϼ���ԭ�������Ĺ�ϵͼ������˵����ȷ����

A��ԭ�Ӱ뾶��Z>Y>X
B����̬�⻯����ȶ��ԣ�R<W
C��WX3��ˮ��Ӧ���ɵĻ����������ӻ�����
D��Y��Z��������������Ӧ��ˮ���������Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com