
��12�֣����ⶨ�Ҵ��Ļ�ѧʽ��C2H6O�������л����ձ����ͬ���칹�����Ʋ��Ҵ��Ľṹ��������������֮һ��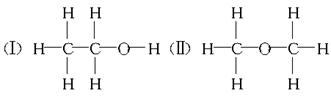
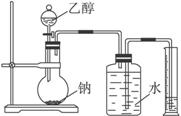
Ϊ�ⶨ��ṹ��Ӧ�������ʵ������Խ��ж��ԡ�����ʵ�顣�ָ����Ҵ����ơ�ˮ����Ҫ����������ס�����λͬѧֱ��������ͼ����װ�ÿ�ʼ����ʵ��ȷ���Ҵ��Ľṹ��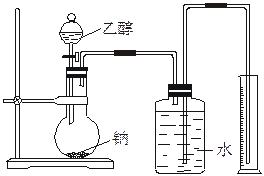
��1��ѧ���õ�һ��ʵ���������ϱ���
| �Ҵ������ʵ���/mol | ���������/L |
| 0.10 | 1.12����״���� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ���ⶨ�Ҵ��Ļ�ѧʽ��C2H6O�������л����ձ����ͬ���칹�����Ʋ��Ҵ��Ľṹ���������ж���֮һ��
���ⶨ�Ҵ��Ļ�ѧʽ��C2H6O�������л����ձ����ͬ���칹�����Ʋ��Ҵ��Ľṹ���������ж���֮һ�� ������
������
| �Ҵ������ʵ�����mol�� | �����������L�� | 0.10 | 1.12����״���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

Ϊ�ⶨ��ṹ��Ӧ�������ʵ������Խ��ж��ԡ�����ʵ�飬�ָ����Ҵ����ơ�ˮ����Ҫ����������ס��ҡ���������ͬѧֱ��������ͼ����װ�ÿ�ʼ����ʵ��ȷ���Ҵ��Ľṹ��
(1)ѧ���õ�һ��ʵ�����ݣ�
�Ҵ������ʵ���/mol | ���������/L |
0.10 | 1.12(��״��) |
�������������ƶ��Ҵ��ĽṹӦΪ________(�â��ʾ)������Ϊ____________________��
(2)ͬѧ�ҷֱ�ȷ����
(3)ͬѧ����Ϊʵ��ɹ��Ĺؼ��У���װ��������Ҫ���� ��ʵ�鿪ʼǰȷȷ���Ҵ����� �������� �ܹ��ƿ��ˮ������� ����������IJ��㷽����ȷ������ȷ��������ȷ����__________��(�����)
(4)ͬѧ������ͨ�������Ҵ���������ȷ���Ҵ���������ô������֪����������__________��
(5)ʵ�������ͬѧ���Ҵ��Ŀ��ܽṹ�������ֶ��Ҵ����Ƶ����Ĺ�ϵ���������ۣ�����Ҵ������ʵ���Ϊn mol����ô���Ƶ����ʵ�����ȡֵҪ�������______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣����ⶨ�Ҵ��Ļ�ѧʽ��C2H6O�������л����ձ����ͬ���칹�����Ʋ��Ҵ��Ľṹ��������������֮һ��
Ϊ�ⶨ��ṹ��Ӧ�������ʵ������Խ��ж��ԡ�����ʵ�顣�ָ����Ҵ����ơ�ˮ����Ҫ����������ס�����λͬѧֱ��������ͼ����װ�ÿ�ʼ����ʵ��ȷ���Ҵ��Ľṹ��
��1��ѧ���õ�һ��ʵ���������ϱ���
| �Ҵ������ʵ���/mol | ���������/L |
| 0.10 | 1.12����״���� |
�������������ƶ��Ҵ��ĽṹΪ ���â��ʾ��������Ϊ
��
�÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��2��ͬѧ�ҷֱ�ȷ����4.60 g �Ҵ����ж��ʵ�飬���������������Ͳ��ˮ�������Ϊ���ɵ�H2���������ɱ�״����С��1.12L�����������Ͳ��������ͬѧ������ɵ�����ô����Ϊ������������Ʒ�к�������ˮ��ɵģ�����Ϊ��ȷ�� �����ȷ������ȷ��������˵�����жϵ����� ��
��3��ʵ�������ͬѧ���Ҵ��Ŀ��ܽṹ�������֣����Ҵ����Ƶ����Ĺ�ϵ���������ۣ�����Ҵ������ʵ���Ϊn mol����ô���Ƶ����ʵ�����ȡֵҪ������� n������ڡ�����С�ڡ����ڡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣����ⶨ�Ҵ��Ļ�ѧʽ��C2H6O�������л����ձ����ͬ���칹�����Ʋ��Ҵ��Ľṹ��������������֮һ��
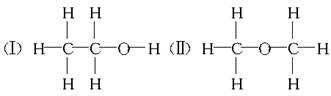
Ϊ�ⶨ��ṹ��Ӧ�������ʵ������Խ��ж��ԡ�����ʵ�顣�ָ����Ҵ����ơ�ˮ����Ҫ����������ס�����λͬѧֱ��������ͼ����װ�ÿ�ʼ����ʵ��ȷ���Ҵ��Ľṹ��

��1��ѧ���õ�һ��ʵ���������ϱ���
|
�Ҵ������ʵ���/mol |
���������/L |
|
0.10 |
1.12����״���� |
�������������ƶ��Ҵ��ĽṹΪ ���â��ʾ��������Ϊ
��
�÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��2��ͬѧ�ҷֱ�ȷ����4.60 g �Ҵ����ж��ʵ�飬���������������Ͳ��ˮ�������Ϊ���ɵ�H2���������ɱ�״����С��1.12L�����������Ͳ��������ͬѧ������ɵ�����ô����Ϊ������������Ʒ�к�������ˮ��ɵģ�����Ϊ��ȷ�� �����ȷ������ȷ��������˵�����жϵ����� ��
��3��ʵ�������ͬѧ���Ҵ��Ŀ��ܽṹ�������֣����Ҵ����Ƶ����Ĺ�ϵ���������ۣ�����Ҵ������ʵ���Ϊn mol����ô���Ƶ����ʵ�����ȡֵҪ������� n������ڡ�����С�ڡ����ڡ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com