
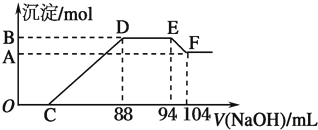
���� ͨ�����⣬��Ӧʼ��û���������ɣ����Եó������е������������ɣ����������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ������Ʋ�NԪ����+5�����-3�ۣ���ͼ�ɵ������������������������ҺӦ�������ᷴӦ�������ɳ�������������ȫ����ͼ֪������������������Һ�����������䣬�ɵ���NH4+�����˷�Ӧ��
��1���ɸ���Al��OH��3 +OH-=AlO2-+2H2O���ó�Al��OH��3�����ʵ�����
��2��DE�η�Ӧ���ӷ���ʽΪ��NH4++OH-�TNH3•H2O�����ͼ�����ݼ�������ɵ�һˮ�ϰ������ʵ�����
��3��Al��OH��3�����ʵ���Ϊ0.05mol��������Ԫ���غ㣬n��Al��=0.05mol�����ݵ���ת���غ����n��Fe��=0.03mol���ݴ��������������������۵����ʵ���֮�ȣ�
��4���������Ϸ�����֪��n��Al��=0.05mol��n��NH4+��=0.03mol�����������ᷴӦʱ������Ļ�ԭ����ΪNH4NO3������������ԭ��Ӧ�غ���д��
��� �⣺ͨ�����⣬��Ӧʼ��û���������ɣ����Եó������е������������ɣ����������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ������Ʋ�NԪ����+5�����-3�ۣ���ͼ�ɵ������������������������ҺӦ�������ᷴӦ�������ɳ�������������ȫ����ͼ֪������������������Һ�����������䣬�ɵ���NH4+�����˷�Ӧ��
��1���ɸ���Al��OH��3 +OH-=AlO2-+2H2O���ó�Al��OH��3�����ʵ���Ϊ����104-94����10-3L��5mol/L=0.05 mol��
�ʴ�Ϊ��0.05��
��2��DE��Ϊ笠����������������ӷ�Ӧ����һˮ�ϰ�����Ӧ���ӷ���ʽΪ��NH4++OH-�TNH3•H2O��������һˮ�ϰ������ʵ���Ϊ����94-88����10-3L��5mol/L=0.03mol��
�ʴ�Ϊ��0.03��
��3��Al��OH��3�����ʵ���Ϊ0.05mol��������Ԫ���غ㣬�ʻ�Ͻ�����n��Al��=0.05mol����ͼ��֪��DE�����ĵ��������Ƶ����Ϊ94mL-88mL=6mL���ʸýβμӷ�Ӧ����������Ϊ0.006L��5mol/L=0.03mol������NH4++OH-�TNH3•H2O ��֪��������Һ��n��NH4+��=0.03ml�����ݵ���ת���غ��У�3n��Fe��+3n��Al��=8n��NH4+������3n��Fe��+3��0.05mol=8��0.03mol����ã�n��Fe��=0.03mol����Ͻ�����n��Al��=0.05mol��n��Fe��=0.03mol����Ʒ�����ۺ����۵����ʵ���֮��Ϊ5��3��
�ʴ�Ϊ��5��3��
��4��������֪�����������ᷴӦʱ������Ļ�ԭ����ΪNH4NO3������������ԭ��Ӧ��ʧ�����غ�͵���غ㣬��Ӧ�����ӷ�ӦΪ��8Al+30H++3NO3-=8Al3++3NH4++9H2O��
�ʴ�Ϊ��8Al+30H++3NO3-=8Al3++3NH4++9H2O��
���� ���⿼�����ﷴӦ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷͼ�����߱仯�ĺ���Ϊ���ؼ���ע�����ճ����������ʼ��仯������е����ʣ�����������ѧ���ķ�����������ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����¶� | B�� | ʹ�ú��ʵĴ��� | ||
| C�� | ��߷�Ӧ��Ũ�� | D�� | �����¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
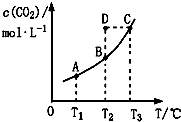
| A�� | �÷�Ӧ�ġ�H��0 | |
| B�� | ��T2ʱ��D��ķ�Ӧ���ʣ��ͣ����������棩 | |
| C�� | A��C������ȣ���������ƽ����Է���������M��A����M��C�� | |
| D�� | ��T1��T2ʱ��ƽ�ⳣ���ֱ�ΪK1��K2����K1��K2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��Ca2+��HCO3-��Br- | B�� | SO32-��Al��OH��4-��K+��Al3+ | ||
| C�� | Fe3+��Na+��NO3-��CO32- | D�� | NH4+��Na+��SiO32-��I- | ||
| E�� | Ca2+��Na+��NO3-��PO43- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����0.1mol•L-1CO32-����Һ��Na+��Al3+��NO3-��S2- | |
| B�� | ��ɫ��Һ��K+��H+��SCN-��SO42- | |
| C�� | $\frac{Kw}{c{��H}^{+}��}$=0.1mol/L����Һ��Na+��K+��HCO3-��NO3- | |
| D�� | ����ˮ�������c��H��=1.0��10-13mol•L-1����Һ�У�Na+��Fe3+��Cl-��CH3COO- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ⶼ�� | B�� | �����ⶼ�� | C�� | �ܢݲ��� | D�� | ȫ����ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����ʹpH��ֽ������Һ�У�Fe2+��Na+��Cl-��NO3- | |
| B�� | �ں���S2-���ӵ���Һ�У�NH4+��K+��Cl-��SO42- | |
| C�� | ����ˮ�������c��H+��=10-12mol•L-1����Һ��Na+��Ba2+��Cl- | |
| D�� | ����ɫ��Һ�У�NH4+��Mg2+��SO42-��SiO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
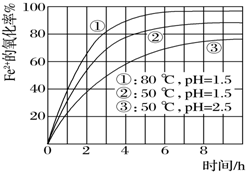 Ϊ���о�һ��Ũ��Fe2+����Һ�ڲ�ͬ�����±����������������ʣ�ʵ������ͼ��ʾ���ж�����˵��������ǣ�������
Ϊ���о�һ��Ũ��Fe2+����Һ�ڲ�ͬ�����±����������������ʣ�ʵ������ͼ��ʾ���ж�����˵��������ǣ�������| A�� | pHԽС������ԽС | |
| B�� | �¶�Խ��������Խ�� | |
| C�� | Fe2+�������ʳ���pH���¶�Ӱ���⣬������������Ӱ�죬��Ũ�ȵ� | |
| D�� | ʵ��˵������pH�������¶����������Fe2+�������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com