
���� ��1�����ݸ��������Һ��ǿ���������и�ʴ�����ܵ�С������ѡ����ʽ�ζ��ܣ������Ĵ�������Һ�����֪ѡ��25ml��ʽ�ζ��ܿ������ʵ��Ҫ��
��2�����������ҺΪ��ɫ��Һ�����Ը�����Һ��ɫ�仯�жϵζ��յ㣬����Ҫָʾ�����ζ�����ǰΪ��ɫ���ζ�������Ϊ�Ϻ�ɫ���ݴ��жϵζ��յ㣻
��3��������Ӧ���̺�ʵ�����ݼ���������������������
��� �⣺��1��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ���KMnO4��ҺӦװ����ʽ�ζ����У��Ĵ�����KMnO4��Һ������ֱ�Ϊ20.00mL��19.98mL��20.02mL��22.00mL������ѡ��25ml��ʽ�ζ��ܿ������ʵ�飬
�ʴ�Ϊ��C��
��2��KMnO4��ҺΪ�Ϻ�ɫ��Һ�����Ը��ݸ��������Һ��Һ��ɫ�仯�жϵζ��յ㣬���Բ���Ҫָʾ�����õζ��յ������Ϊ���������һ������KMnO4��Һ����Һ����ɫ��Ϊ�Ϻ�ɫ��30���ڲ��ָ���˵����Ӧ������
�ʴ�Ϊ���� �������һ������KMnO4��Һ����Һ����ɫ��Ϊ�Ϻ�ɫ��30���ڲ��ָ���
��3������������Һ�й�����������=5.0ml��1.0g/ml=5g����0.1000mol/L KMnO4��Һ�ζ�����ͬ�������ζ����Ĵ�����KMnO4��Һ������ֱ�Ϊ20.00mL��19.98mL��20.02mL��22.00ml�����Ĵ����ϴ���ȥ��ƽ�� ���ĸ��������Һ���=$\frac{20.00+19.98+20.02}{3}$=20.00mL��
���ݷ�Ӧ 2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
2 5
0.2000mol/L��0.02L n��H2O2��
$\frac{2}{0.2000mol/L��0.02L}$=$\frac{5}{n��{H}_{2}{O}_{2}��}$����ã�n��H2O2��=0.01mol��
�������������������$\frac{34g/mol��0.01mol}{1.00g/mL��10.00mL}$��100%=3.40%��
�ʴ�Ϊ��3.40%��
���� ���⿼�����к͵ζ�����Ŀ�ѶȲ�����ȷ��Ӧԭ��Ϊ���ؼ���ע�����������к͵ζ���������������������ѧ���ķ�����������������ѧʵ��������


 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
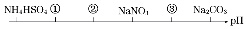 ��ͬ�¶ȣ���ͬŨ���µ�������Һ����pH��С�����˳����ͼ��ʾ��ͼ�Т٢ڢ۴��������ʿ��ܷքeΪ��������
��ͬ�¶ȣ���ͬŨ���µ�������Һ����pH��С�����˳����ͼ��ʾ��ͼ�Т٢ڢ۴��������ʿ��ܷքeΪ��������| A�� | NH4Cl����NH4��2SO4��CH3COONa | B�� | ��NH4��2SO4��NH4Cl��CH3COONa | ||
| C�� | ��NH4��2SO4��NH4Cl��NaOH | D�� | CH3COONa��NH4Cl����NH4��2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ʹ���Ը��������Һ��ɫ | |
| B�� | һ�ȴ������ֻ��һ�� | |
| C�� | ���ӽṹ�м�����Ŀ������0��1��2 | |
| D�� | ������CH4��C4H8�ֱ�����������ȫȼ�գ�CH4�ĺ�����С��C4H8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.01 mol•L-1HA����Һ�� c��H+��=l��l0-4mol•L-1 | |
| B�� | pH=3��HA��Һ��pH=ll��NaOH��Һ�������Ϻ�������Һ�У�c��Na+��=c��A-����c��OH-��=c��H+�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��HA��Һ��NaA��Һ�������Ϻ�������Һ�����ԣ���c��H+��-c��OH-����c��A-��-c��HA�� | |
| D�� | pH=3��HA��Һ��pH=11��NaOH��Һ��Ϻ�������Һ�Լ��ԣ��� c��Na+����c��A-����c��OH-������c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
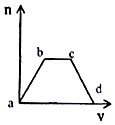 ������CO2ͨ��KOH��Ca��OH��2�Ļ����Һ�У����ɳ�����������m����ͨ��CO2�����V���Ĺ�ϵ��ͼ��ʾ��
������CO2ͨ��KOH��Ca��OH��2�Ļ����Һ�У����ɳ�����������m����ͨ��CO2�����V���Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���

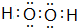 ��������E2 D2�뻯����E2H��ˮ��Һ�����ʵ�����1��1��Ӧ���ɵ���H�����ӷ���ʽΪNa2O2+2H2O+S2-=S+4Na++4OH-
��������E2 D2�뻯����E2H��ˮ��Һ�����ʵ�����1��1��Ӧ���ɵ���H�����ӷ���ʽΪNa2O2+2H2O+S2-=S+4Na++4OH-�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com