
����Ŀ���к͵ζ��ǻ�ѧ����ʵ��֮һ��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ������д���пհף�
��1�����к͵ζ��Ĺ����������²��������ñ���Һ��ϴ�ζ��� �����ζ�����ע�����Һ �����ζ����Ƿ�©ˮ ���ζ������ڲ�����������ȷ��˳����__________________����д��ţ�
��2����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е�________��Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��

��3��ѡ�õ�ָʾ����_____________________ ��(a��ʯ�� b����̪)
��4���ñ���������Һ�ζ����������������Һʱ�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��______________________��
��5�����в����п���ʹ��������������Һ��Ũ����ֵƫ�͵���_______________��
A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��6��������±������ݼ��������������Һ�����ʵ���Ũ�ȣ�c(NaOH)�� ________________������ȷ��С�������λ��
�ζ����� | ��������������Һ�����/ mL | 0.1000 mol/L ��������/ mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ���/ mL | ||
��һ�� | 25.00 | 0.00 | 26.10 | 26.10 |
�ڶ��� | 25.00 | 2.00 | 28.08 | 26.08 |
������ | 25.00 | 0.22 | 26.34 | 26.12 |
��7���ζ��յ���ж�������_____________________________________________��
���𰸡��ۢ٢ڢ� �� b ��ƿ����Һ��ɫ�ı仯 D 0.1044mol/L �������һ������ʱ����Һǡ���ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���ԭ������ɫ
��������
��1���к͵ζ��IJ�������Ϊ���ۼ��ζ����Ƿ�©ˮ�����ñ���Һ��ϴ�ζ��ܣ������ζ�����ע�����Һ���ܵζ���������ȷ�ĵζ�˳��Ϊ���ۢ٢ڢܣ��ʴ�Ϊ���ۢ٢ڢܣ�
��2����ȥ��ʽ�ζ��������ݵķ���Ӧ���ñ���Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ���ʴ�Ϊ������
��3���������������ǡ�÷�Ӧ��Һ�����ԣ���ѡ���̪�������ָʾ������ѡb���ʴ�Ϊ��b��
��4������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯���ʴ�Ϊ����ƿ����Һ��ɫ�ı仯��
��5��A���ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��ϡ�ͣ���Һ��Ũ��ƫС�����V������ƫ�ⶨc�����⣩ƫ��A����
B��ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬�ⶨc�����⣩��Ӱ�죬��B����
C���ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ�ⶨc�����⣩ƫ��C����
D�ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС���ⶨc�����⣩ƫ�ͣ���D��ȷ��
��ѡD���ʴ�Ϊ��D��
��6�����εζ��������������ֱ�Ϊ26.10mL��26.08mL��26.12mL���������ݾ���Ч��ƽ������V�����ᣩ=26.10mL���ɻ�ѧ����ʽ�ɵã�0.02610L��0.1000mol/L=0.025L��c��NaOH������c��NaOH��= =0.1044mol/L,�ʴ�Ϊ��0.1044mol/L��
��7���ζ��յ���ж���������Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ɫ���ʴ�Ϊ����Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ɫ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�����������ԭ�Ӷ����������Ϊ8���ӽṹ����( )
A. BF3B. CCl4C. SCl6D. HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�����ر����������������ó�ʪ������������ƿ��ͨ����ƿ����������ɫ�����ر���������������ͨ���������壬������ɫ����ƿ����ʢ���Լ���������

A. ŨH2SO4B. NaOH��ҺC. NaCl��ҺD. Ca(OH)2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����������(NiC2O4��2H2O)������ˮ����ҵ�ϴӷ�������(��Ҫ�ɷ�ΪNi������һ������Al2O3��FeO��SiO2��CaO��)�Ʊ������������������ͼ��ʾ��

��֪������ؽ������������������������pH���±���
�������� | Fe3+ | Fe2+ | Al3+ | Ni2+ |
��ʼ������pH | 1.1 | 5.8 | 3.0 | 6.8 |
��ȫ������pH | 3.2 | 8.8 | 5.0 | 9.5 |
��Ksp(CaF2)=1.46��10��10
�۵�ij����Ũ��С��1.0��10��5mol��L��1ʱ����Ϊ��ȫ������
��1����д��һ�����������������ʵĴ�ʩ��_________________________________��
��2���Լ�a��һ����ɫ��������д����������ʱ��Ӧ�����ӷ���ʽ______________��
��3��pH�ĵ��ط�ΧΪ__________��
��4��д�������ơ�ʱ�����ӷ�Ӧ����ʽ��_________________________________����Ca2+������ȫʱ����Һ��F��Ũ�ȵ���СֵΪ___________mol��L��1(д������ʽ����)��֤��Ni2+�Ѿ�������ȫ��ʵ�������������_________��
��5������a��������___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��װ���з�����Ӧ2A2(g)+B2(g)![]() 2C(g) ��H=��a kJ��mol1��a>0������֪P�ǿ����ɻ����Ļ���������ͬ�¶��¹ر�K����A��B�����зֱ����2mol A2��1mol B2���������ֱ���500��ʱ�ﵽƽ�⣬A��C��Ũ��Ϊ
2C(g) ��H=��a kJ��mol1��a>0������֪P�ǿ����ɻ����Ļ���������ͬ�¶��¹ر�K����A��B�����зֱ����2mol A2��1mol B2���������ֱ���500��ʱ�ﵽƽ�⣬A��C��Ũ��Ϊ![]() mol��L-1���ų�����b kJ��B��C��Ũ�ȷֱ�Ϊ
mol��L-1���ų�����b kJ��B��C��Ũ�ȷֱ�Ϊ![]() mol��L-1���ų�����c kJ����ش��������⣺
mol��L-1���ų�����c kJ����ش��������⣺

��1�������¶����ߵ�700������Ӧ��ƽ�ⳣ����_____������������������С������������������
��2���Ƚϴ�С����1_____��2������>������=��������<������a��b��c�ɴ�С�Ĺ�ϵΪ_____��
��3������K��һ��ʱ������´ﵽƽ�⣬����B�������_____������������������С������������������
��4������A��B�������ҹ̶�P����B�иij���4 mol A2��2 mol B2����500��ʱ�ﵽƽ���C��Ũ��Ϊ��3 mol��L��1������1����3�Ĺ�ϵΪ_____��
��5��ʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����_____��
a����ʱ�����C���� b���ʵ������¶� c������B2��Ũ�� d��ѡ���Ч�Ĵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���в���Ҫʹ���¶ȼƵ���
A. ��KNO3�ܽ�ȵ�ʵ��B. ʵ�������屽
C. ʯ�͵ķ���D. ʵ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2 L�ܱ������н��з�Ӧ��mX(g)��nY(g)![]() pZ(g)��qQ(g)��ʽ��m��n��p��qΪ��ѧ����������0��3 min�ڣ����������ʵ����ı仯���±���ʾ��
pZ(g)��qQ(g)��ʽ��m��n��p��qΪ��ѧ����������0��3 min�ڣ����������ʵ����ı仯���±���ʾ��

��֪2 min��v(Q)��0.075 mol��L��1��min��1��v(Z)��v(Y)��1��2��
��1����ȷ������ʼʱn(Y)��________��n(Q)��________��
��2������ʽ��m��________��n��________��p��________��q��________��
��3����Z��ʾ2 min�ڵķ�Ӧ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2��3-�����(![]() )�����ڵ��ӻ�ѧƷ�������У���ϳ�·�����£�
)�����ڵ��ӻ�ѧƷ�������У���ϳ�·�����£�
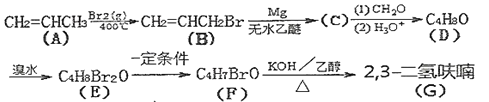
��֪��
�ش��������⣺
(1)A��B�ķ�Ӧ����Ϊ___________��
(2)A������Ϊ___________��B���������ŵ�����Ϊ___________��
(3)д��E�Ľṹ��ʽ______________________��
(4)д��F��G�Ļ�ѧ����ʽ______________________��
(5)��������������F��ͬ���칹�干��___________�֡�
����![]() ; ��������״�ṹ ; �������������칹��
; ��������״�ṹ ; �������������칹��
(6)д����������������D��ͬ��������Ľṹ��ʽ___________��
���ܷ���������Ӧ�����˴Ź������������ַ��ҷ����֮��Ϊ6�U1�U1��
(7)д����1��3-����ϩΪԭ���Ʊ������(![]() )�ĺϳ�·��_____________________(���Լ���ѡ)��
)�ĺϳ�·��_____________________(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�����~����ʾԪ�����ڱ��IJ���Ԫ�ء�
IA | IIA | IIIA | IVA | VA | VIA | VIIA | |
1 | |||||||
2 | �� | �� | �� | ||||
3 | �� | �� | �� | �� |
��1����Ԫ�������������ȴ�����������______������Ԫ�صķ�����_______����Ԫ�ص��⻯��ĵ���ʽΪ____________________��
��2�����٢ۢ�����Ԫ����ɵ�������______________�������ʵ�ˮ��Һ��_____�ԡ�
��3����Ԫ�ص�ԭ�Ӱ뾶��������������____________________________________��
��4����Ԫ�ص�����������Ӧ��ˮ����ļ���ǿ����Ԫ�أ���һ����ѧ����ʽ��֤����________________________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com