
����Ŀ������[KAl(SO4)2��12H2O]���������������й㷺��;������ˮ�ľ�������ֽ��ҵ����ʩ������ʳƷ��ҵ�ķ��ͼ��ȡ������������ķ�����������(��Al��Al2O3������SiO2��FeO��xFe2O3)���Ʊ������������������£��ش��������⣺
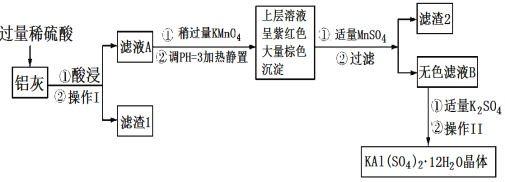
��1��������ˮ��ԭ����______________(�����ӷ���ʽ��ʾ)��
��2����������________��������������Ũ����__________�����ˡ�ϴ�ӡ����
��3��������ҺA���Ƿ����Fe2�����Լ���__________(ֻ��һ���Լ�)��
��4��������Ͷ������������Һ����������Ļ�ѧ����ʽ��__________������ҺA�м��������ط�����Ӧ�����ӷ���ʽΪ(��������MnO4-ת��ΪMn2��)��_______��
��5����֪����pH��3�����������£�MnO4-����Mn2����Ӧ����MnO2������MnSO4������Ӧ�����ӷ���ʽΪ��________������2���е�������________��
���𰸡�Al3����3H2O![]() Al(OH)3(����)��3H�� ���� ��ȴ�ᾧ ���Ը��������Һ(�����軯����Һ) 2Al��2NaOH��2H2O=2NaAlO2��3H2�� 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O 3Mn2����2MnO4-��2H2O=5MnO2����4H�� MnO2��Fe(OH)3
Al(OH)3(����)��3H�� ���� ��ȴ�ᾧ ���Ը��������Һ(�����軯����Һ) 2Al��2NaOH��2H2O=2NaAlO2��3H2�� 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O 3Mn2����2MnO4-��2H2O=5MnO2����4H�� MnO2��Fe(OH)3
��������
��1��������ǿ�������Σ�![]() ˮ����������������壬�ܹ�����ˮ�������������γɳ�������ȥ���Ӷ��ﵽ��ˮ��Ŀ�ġ��䷴Ӧԭ���÷���ʽ��ʾ��Al3����3H2O
ˮ����������������壬�ܹ�����ˮ�������������γɳ�������ȥ���Ӷ��ﵽ��ˮ��Ŀ�ġ��䷴Ӧԭ���÷���ʽ��ʾ��Al3����3H2O![]() Al(OH)3(����)��3H����
Al(OH)3(����)��3H����
��2���������ǽ������Թ�������Һ����IJ������й��ˡ�������������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����͵õ�������
�ʴ𰸣����ˣ���ȴ�ᾧ��
��3��������ҺA���Ƿ����![]() �ķ����������軯����Һ���������ɫ������֤������
�ķ����������軯����Һ���������ɫ������֤������![]() ���ʴ𰸣����軯����Һ��
���ʴ𰸣����軯����Һ��
��4��2Al��2NaOH��2H2O=2NaAlO2��3H2����Al2O3+2OH-=2AlO2-+H2O������ҺA�м��������ص�Ŀ����ʹ![]() ת��Ϊ
ת��Ϊ![]() ��������Ӧ�����ӷ���ʽΪ5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O��
��������Ӧ�����ӷ���ʽΪ5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O��
��5�����ݱ������ݿ�֪����Һ��pH=3ʱ![]() �����γɳ���
�����γɳ���![]() ��������ɵ÷���ʽ3Mn2����2MnO4-��2H2O=5MnO2����4H����������Һ��pH=3�����������ijɷֺ���MnO2��Fe(OH)3��
��������ɵ÷���ʽ3Mn2����2MnO4-��2H2O=5MnO2����4H����������Һ��pH=3�����������ijɷֺ���MnO2��Fe(OH)3��


 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ����������������� ��
A.������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС
B.��ˮ����������Һ����ƽ��δ��ȴ����������������������������ƫ��
C.��ȡ2.3gNaCl���壬�������������ƽ����ߣ�����������ҩƷ����ƫС
D.����Ͳ��ȡ5.0mLŨ���ᣬ���Ӷ��������õ�Ũ��������ƫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2 mol SO2��1 mol O2����һ�ݻ��̶����ܱ������У���һ�������·�����Ӧ��2SO2(g)��O2(g) ![]() 2SO3(g)���������������ٸı�ʱ��������˵����ѧ��Ӧ�Ѵﵽƽ��״̬����(����)
2SO3(g)���������������ٸı�ʱ��������˵����ѧ��Ӧ�Ѵﵽƽ��״̬����(����)
A. ���������ܶ�
B. ��������ѹǿ
C. �������������ʵ���
D. ��������ƽ����Է�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ 3.6 g ̼�� 6.4 g ��������ȼ�գ�����Ӧ��ľ������ų� X kJ ��������֪���� C(s)��ȼ������ֵΪ Y kJ��mol��1���� 1 mol C(s)�� O2(g)��Ӧ���� CO(g)�ķ�Ӧ�� ��H Ϊ�� ��
A. -Y kJ/mol B. -(10X��Y) kJ/mol C. -(5X��0.5Y) kJ/mol D. +(10X��Y) kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������18.4 mol/L��Ũ![]() ������500 mL 0.2 mol/L��ϡ
������500 mL 0.2 mol/L��ϡ![]() ���ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� �ܽ�ͷ�ι� ����Ͳ ��������ƽ ��ҩ�ס���ش��������⣺
���ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� �ܽ�ͷ�ι� ����Ͳ ��������ƽ ��ҩ�ס���ش��������⣺
��1�����������У�������ϡ![]() ʱ����Ҫʹ�õ���________________������ţ�����ȱ�ٵ�������________________��
ʱ����Ҫʹ�õ���________________������ţ�����ȱ�ٵ�������________________��
��2�������㣬��Ũ![]() �����Ϊ________����ȡŨ����ʱӦѡ��________��ѡ���10 mL����50 mL����100 mL���ֹ����Ͳ��
�����Ϊ________����ȡŨ����ʱӦѡ��________��ѡ���10 mL����50 mL����100 mL���ֹ����Ͳ��
��3������һ�����ʵ���Ũ��NaOH��Һ��ʵ���У�����������²�����
A������������������룻 B����NaOH����ֽ���ϳ�����
C������ʱ���ӿ̶��� D��������ƿת��ʱ��������Һ�彦��
E��δϴ���ܽ�NaOH���ձ� F������ƿδ���T����������Һ
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ�δ��ˮ���̶��ߡ�
H��δ��ȴ�����¾ͽ��ж��� I������ʱ������ˮ��������ƿ��
�����Ƶ���Һ���ʵ���Ũ�ȴ�С������ɵ�Ӱ���ǣ���д��ĸ��ƫ�����_________��ƫС����__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ����ͼ��ʾ(ͼ�еĦ�H��ʾ����1 mol���������)��������ͼ�ش��������⣺
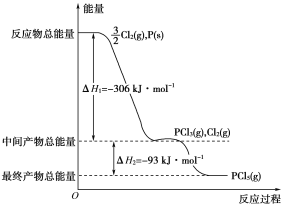
��1��P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽ��__________________________
��2��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ��_________________________
��3��P��Cl2��������Ӧ����1 mol PCl5�Ħ�H3��________��P��Cl2һ����Ӧ����1 mol PCl5�Ħ�H4______��H3(����ڡ���С�ڡ����ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ʵ��װ�õ�˵������ȷ����
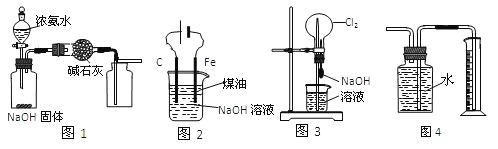
A. ��ͼ1װ����ȡ���﴿����NH3
B. ��ͼ2װ���Ʊ�Fe(OH)2���ܽϳ�ʱ��۲�����ɫ
C. ��ͼ3װ�ÿ����������Ȫ��ʵ��
D. ��ͼ4װ�ò���Cu��Ũ���ᷴӦ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijһ�������Է���мΪԭ���Ʊ�FeCl3��Һ������ӡˢ��·ͭ�帯ʴ����������ҺB���е�����ʵ��������ͼ��

(1)�Լ�aӦѡ��________(��д����)��
(2)������õ�����Ҫ����������©����________(��д��������)��
(3)д�����������Ҫ��Ӧ�Ļ�ѧ����ʽ____________________��
(4)ʵ������ȡ����E�����ӷ���ʽ��________________________����������E���и�������գ���ѡ������װ���е�________(��д���)��

(5)����û�ѧ������������E��______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���ǿ�����ȡ�����ᣬ���Ǹ��ֽⷴӦ�Ĺ���֮һ����֪20��ʱ��K(C6H5OH)=1.2��10-10��K(CH3COOH)=1.8��10-5��K(H2CO3)=4.3��10-7��K(HCO3-)=5.6��10-11��K(HCN)=4.9��10-10��
(1)�����������Ϣ��Na2CO3+C6H5OH![]() NaHCO3+C6H5ONa�Ļ�ѧƽ�ⳣ��K=__________��
NaHCO3+C6H5ONa�Ļ�ѧƽ�ⳣ��K=__________��
(2)������ij������ܽ���ˮ�к���Һ�е�c(H+)=10-9molL-1����õ���ʿ�����_________(�����)��
A��CuSO4 B��HCl C��Na2S D��NaOH E��K2SO4
(3)�����£���pH=3������a L�ֱ�������������Һ��ϣ������Һ�������ԡ���Ũ��Ϊ1.0��l0-3mol��L-1�İ�ˮb L����c(OH-)=1.0��10-3mol��L-1�İ�ˮc L����c(OH-)=1.0��10-3molL-1������������Һd L����a��b��c��d֮���ɴ�С�Ĺ�ϵ��_________��
(4)����ʱ����0.2molL-1�Ĵ�����Һ�м���������0.1molL-1��NaOH(aq)����ַ�Ӧ��������Һ��pH=4����������Һ�и�����Ũ�ȴ�С��ϵ��________________��������Һ�е������غ�ʽΪ��________+________=__________=_________ molL-1��������Һ��c(CH3COOH)=____________________molL-1(д����ʽ����������)��
(5)��֪����ʱKsp(AgCl)=1.8��10-10mol2L-2����50mL 0.018molL-1��AgNO3��Һ�м�����ͬ���0.020molL-1�����ᣬ��c(Ag+)=_______________����ʱ���û����Һ��pH=_____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com