
����Ŀ������18.4 mol/L��Ũ![]() ������500 mL 0.2 mol/L��ϡ
������500 mL 0.2 mol/L��ϡ![]() ���ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� �ܽ�ͷ�ι� ����Ͳ ��������ƽ ��ҩ�ס���ش��������⣺
���ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� �ܽ�ͷ�ι� ����Ͳ ��������ƽ ��ҩ�ס���ش��������⣺
��1�����������У�������ϡ![]() ʱ����Ҫʹ�õ���________________������ţ�����ȱ�ٵ�������________________��
ʱ����Ҫʹ�õ���________________������ţ�����ȱ�ٵ�������________________��
��2�������㣬��Ũ![]() �����Ϊ________����ȡŨ����ʱӦѡ��________��ѡ���10 mL����50 mL����100 mL���ֹ����Ͳ��
�����Ϊ________����ȡŨ����ʱӦѡ��________��ѡ���10 mL����50 mL����100 mL���ֹ����Ͳ��
��3������һ�����ʵ���Ũ��NaOH��Һ��ʵ���У�����������²�����
A������������������룻 B����NaOH����ֽ���ϳ�����
C������ʱ���ӿ̶��� D��������ƿת��ʱ��������Һ�彦��
E��δϴ���ܽ�NaOH���ձ� F������ƿδ���T����������Һ
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ�δ��ˮ���̶��ߡ�
H��δ��ȴ�����¾ͽ��ж��� I������ʱ������ˮ��������ƿ��
�����Ƶ���Һ���ʵ���Ũ�ȴ�С������ɵ�Ӱ���ǣ���д��ĸ��ƫ�����_________��ƫС����__��
���𰸡��ڢޢ� 500 mL����ƿ 5.4 mL �� AH BCDE
��������
��ʵ����������500 mL 0.2 mol/L��ϡ������Ҫ�õ��IJ�����������Ͳ���ձ�������������ͷ�ιܺ�500ml����ƿ��
��2������ϡ�Ͷ��ɼ���ɵ�Ũ����������������ȡ���ԭ��ѡ����Ͳ��
��3������c=![]() ������Һ��������ʵ����ʵ����Ƿ����仯�������
������Һ��������ʵ����ʵ����Ƿ����仯�������
��1������500 mL0.2 mol��L-1��������Һ��Ҫ����������Ͳ���ձ�����������500ml����ƿ�ͽ�ͷ�ιܣ������������֪��������ϡ![]() ʱ����Ҫʹ�õ�����ƿ��������ƽ ��ҩ�ף�����Ҫ��������500 mL����ƿ���ʴ�Ϊ���ڢޢ���500 mL����ƿ��
ʱ����Ҫʹ�õ�����ƿ��������ƽ ��ҩ�ף�����Ҫ��������500 mL����ƿ���ʴ�Ϊ���ڢޢ���500 mL����ƿ��
��2������ҪŨ��������ΪV mL����ϡ�Ͷ��ɿ�֪��ϡ��ǰ����������ʵ������䣬���У�V��10-3L��18.4 mol/L =0.5 L��0.2 mol/L����ã�V��5.4������ȡҺ�����ԭ���֪Ӧѡ��10 mL��Ͳ��ȡŨ���ᣬ�ʴ�Ϊ��5.4����
��3��A��������������������ᵼ�³�����NaOH������ƫ����Һ���������Ƶ����ʵ�������������ҺŨ�Ƚ�ƫ�ߣ�
B����NaOH����ֽ���ϳ������������ƻ����տ����еĶ�����̼��ˮ���ᵼ��NaOH�����ʵ�����С��������ҺŨ�Ƚ�ƫ�ͣ�
C������ʱ��������ƿ�̶��ߣ���ʹ��Һ���ƫ�����Ũ��ƫС��
D��������ƿת��ʱ��������Һ�彦����ʹ����NaOH���ʵ�����С�����Ũ��ƫС��
E��δϴ���ܽ�NaOH���ձ�����ʹNaOH���ʵ�����С�����Ũ��ƫС��
F������ƿδ���T����������Һ������ʱ��Ҫ��������ˮ����ϡ�Ͷ��ɿ�֪��ϡ��ǰ��NaOH�����ʵ������䣬������ǰ������ƿ������������ˮ��������ҺŨ����Ӱ�죻
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ�δ��ˮ���̶��ߣ���Һ����ޱ仯����������ҺŨ����Ӱ�죻
H��δ��ȴ�����¾ͽ��ж��ݣ����ܽ�ų���������ȴ�����º�ʹ��Һ���ƫС�����Ũ��ƫ��
I������ʱ������ˮ��������ƿ�⣬������ƿ����Һ�����Ӱ�죬��������ҺŨ����Ӱ�죻
��ƫ�����AH��ƫС����BCDE����Ӱ�����FGI���ʴ�Ϊ��AH��BCDE��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ������ȡ������������ͭ��ʵ�飬����ʵ�鲽�裬�ش��й����⡣

��1��������ƽ���ʱ����ָ��ƫ���ұߣ�Ӧ����ߵ���˿____________����������ҡ���������
��2���μ�NaOH��Һ�����ɳ��������ӷ���ʽΪ_______________________________��
��3����������������Ҫ�õ�����������������_________________________________��
��4��ϴ�Ӹó����ķ�����______________________________________________________��Ϊ�˼���˳����Ƿ�ϴ�Ӹɾ���Ӧȡ���һ�ε�ϴ��Һ����������__________��Һ���顣
��5������CuSO4��Һ�еμ���100mLNaOH��Һ����NaOH��Һ�����ʵ���Ũ������Ϊ_________��
��6���������������Ƶ�CuO������Ϊ _______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й����ʵ�ת����ϵ����ͼ��ʾ(�������ʺͷ�Ӧ��������ȥ)��A������������ʹ����㷺�Ľ������ʣ�B��һ����ɫ��ζ��Һ�壬D��һ�ִ������������G�Ļ���ʻ�ɫ��F��һ�ֻ���ɫ���壬K��һ��Ԫ�صĻ��ϼ�Ϊ��5��
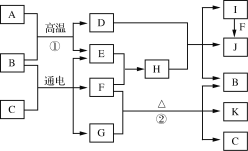
(1) I�Ļ�ѧʽΪ______��
(2) G�ĵ���ʽΪ ______��
(3) д����Ӧ�ٵĻ�ѧ����ʽ��________________��
(4) д����Ӧ�ڵ����ӷ���ʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������Mg2+��NH4+��Al3+�����Һ�м���Na2O2���������ɳ������������(������)�Ĺ�ϵ��ͼ��ʾ������Һ��Mg2+��NH4+��Al3+�������ӵ����ʵ���֮��Ϊ( )
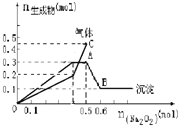
A. 1��1��2B. 2��2��1
C. 1��2��2D. 9��2��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڸ��������£�����ѡ����ʾ�����ʼ�ת������ʵ�ֵ���
A. NaHCO3(s) ![]() Na2CO3(s)
Na2CO3(s) ![]() NaOH(aq)
NaOH(aq)
B. Al(s)![]() NaAlO2(aq)
NaAlO2(aq)![]() Al(OH)3(s)
Al(OH)3(s)
C. AgNO3(aq)![]() [Ag(NH3)2]+(aq)
[Ag(NH3)2]+(aq)![]() Ag(s)
Ag(s)
D. Fe2O3(s)![]() Fe(s)
Fe(s)![]() FeCl3(aq)
FeCl3(aq)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������[KAl(SO4)2��12H2O]���������������й㷺��;������ˮ�ľ�������ֽ��ҵ����ʩ������ʳƷ��ҵ�ķ��ͼ��ȡ������������ķ�����������(��Al��Al2O3������SiO2��FeO��xFe2O3)���Ʊ������������������£��ش��������⣺
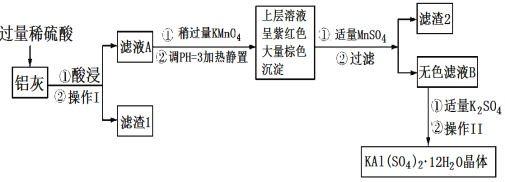
��1��������ˮ��ԭ����______________(�����ӷ���ʽ��ʾ)��
��2����������________��������������Ũ����__________�����ˡ�ϴ�ӡ����
��3��������ҺA���Ƿ����Fe2�����Լ���__________(ֻ��һ���Լ�)��
��4��������Ͷ������������Һ����������Ļ�ѧ����ʽ��__________������ҺA�м��������ط�����Ӧ�����ӷ���ʽΪ(��������MnO4-ת��ΪMn2��)��_______��
��5����֪����pH��3�����������£�MnO4-����Mn2����Ӧ����MnO2������MnSO4������Ӧ�����ӷ���ʽΪ��________������2���е�������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и���Һ�У�Na+���ʵ���Ũ�������ǣ�������
A. 4 L��0.5 mol��L-1NaCl��Һ B. 1 L��0.3 mol��L-1Na2SO4��Һ
C. 5 L��0.4 mol��L-1NaOH��Һ D. 2 L��0.15 mol��L-1��Na3PO4��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ߴ�������[Sr(NO3)2]���������������ܡ���ѧ������Ҳ����ҽҩ�ȡ�
I.��ҵ�������г���������ơ����ᱵ������(����ƿ�����Ũ���ᣬ�������ȡ����ᱵ������Ũ����)���ᴿ�����ȵ�ʵ�鲽�����£�
��ȡ�����ʵ���������Ʒ��__________�����裬���ˣ�����ŨHNO3ϴ��������
�ڽ���������ˮ�У����Թ���(NH4)2Cr2O7(�ظ����)���ð�ˮ����pHΪ7.5���ң�ʹBa2�����������ˡ�
�۽���Һ���������pHΪ1ʱ����������H2C2O4��2H2O��������Cr2O72-��ԭΪCr3+�����ð�ˮ����pH=7������Cr(OH)3���������ˡ�
�ܽ���Һ���������pH��2��3������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӡ�
�ݽ��õ���Sr(NO3)2��2H2O������100 �������¸���õ��ߴ������ȡ�
(1)������������٣�
(2)�������H2C2O4��2H2O��������Cr2O72-��ԭΪCr3+��ͬʱ����һ����ɫ��ζ�����壬д���÷�Ӧ�����ӷ���ʽ��__________��
(3)����������ɵ�Cr(OH)3���������������ѭ��ʹ�ã������ǣ�һ����������Cr(OH)3�м���H2O2���ټ��백ˮ�����ɵõ�(NH4)2Cr2O7(�ظ����)����д����Cr(OH)3����(NH4)2Cr2O7�Ļ�ѧ����ʽ��_____________��
(4)��������(SrO2��2H2O)�Ʊ�ԭ����Sr(NO3)2+H2O2+2NH3��H2O=SrO2��2H2O��+2NH4NO3�������ͬʱ���� H2O2 ����������Ũ�ȵı仯��ϵ��ͼ��ʾ��5%��H2O2 �� 20%��H2O2 �������ʵ͵�ԭ���� _____________��

II.Sr(NO3)2�����ֽ⣬����Sr(NO2)2��O2����500 ��ʱ��Sr(NO2)2��һ���ֽ�����SrO���������ȡһ��������Sr(NO2)2��Sr(NO3)2��Ʒ����������ȫ�ֽ⣬�õ�10.40 g SrO�����10.16 g������塣�������Ʒ��Sr(NO3)2����������(��д��������̣������ȷ��0.01)____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����V L HCl����(��״��)���ܽ���1 Lˮ(ˮ���ܶ�Ϊ1 g/cm3)�У��γɱ�����Һ��������Һ���ܶ�Ϊ�� g/mL����������Ϊ�������ʵ���Ũ��Ϊc mol/L���ܽ��Ϊs g�����й�ϵʽ����ȷ����( )
A.![]() B.
B.![]() C.
C.![]() D.
D.![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com