
������ֵ��ʹ1 g��֬��������Ҫ���������ص�������mg����
�ڵ�ֵ��ʹ100 g��֬�ӳ�ʱ���ĵ��ʵ��������g����
�۸�����֬������ֵ����ֵ�б����£�
| ������ | �������� | ţ�� | ���� | Ӳ������ | ���� |
����ֵ | 190 | 180 | 192 | 226 | 193 | 193 |
��ֵ | 90 | 182 | 38 | 38 | 5 | 126 |
��1�������ͣ�C17H33COO��3C3H5����Է�������884���γɵ��ͣ���������������ʱ������ֵΪ_________��д���䷴Ӧ����ʽ��________________________________________��
��2�������Т١��۵������������ʵ��Ĵʾ䡣
���������ͱȻ�����������_________�ࣻ�ڻ��ͱ�ţ��������_________�ࣻ��Ӳ�����͵ĵ�ֵС��ԭ����_________________________________________��
��3��Ϊʹ��ֵΪ180��100 g����Ӳ�������������������ڱ�״����Ϊ����L��
��4�������нṹʽ������������������ֵΪ430����nΪ���٣���������淴Ӧ����ʽ��
![]()
��1��190 (C17H33COO)3C3H5+3KOH![]() C3H5(OH)3+3C17H33COOK
C3H5(OH)3+3C17H33COOK
��2���ٲ��������� �ڵͼ�֬������ �۲����ͼ���
��3��15.9 L
��4��n=4 C4H9COOK C2H5OH
��������1���ݷ�Ӧ��
(C17H33COO)3C3H5+3KOH![]() 3C17H33COOK+C3H5(OH)3
3C17H33COOK+C3H5(OH)3
������ֵΪx����
884 g��(3��56��103) mg=1 g��x����x=190 mg
��2����������ɵã���֬ʽ��ԽС������ֵԽ����֬ʽ��Խ������ֵԽС������ֵԽ�����ͼ���ĿԽ�ࣻ��ֵԽС�������ͼ���ĿԽ�٣�����������ϵ���ѵõ����ٲ�����֬�������ڸ�֬����۲����ͼ��١�
��3��127 g����1 g H2����״�������Ϊ11.2 L��Ϊ�����ʵ�����������H2�����ΪV����
V=![]()
��4�������ķ���ʽΪCn+3H2n+6O2����Է�������Ϊ��12n+36+2n+32+6=14n+74��KOH����Է�������Ϊ56���ù�ϵʽ��14n+74����56=100��43��n=4��
��ӦʽΪ��
C4H9COOC2H5+KOH![]() C4H9COOK+C2H5OH
C4H9COOK+C2H5OH


 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
�ο����Т١�����ش����⡣
������ֵ��ʹ1g��֬��������Ҫ���������صĺ�������
�ڵ�ֵ��ʹ100g��֬�ӳ�ʱ���ĵ��ʵ�Ŀ�����
�۸�����֬������ֵ����ֵ�б����£�
���������������� �������������� ������������ ţ�������������� ������������ Ӳ���������� ����
����ֵ�������� 190������������ 180������������ 192������������ 226�������� 193���������������� 193
�� ֵ�������� 90���������������� 182������������ 38���������������� 38������������ 5�������������������� 126
��1��������(C17H33COO)3C3H5����Է�������884���γɵ��ͣ���������������ʱ������ֵΪ__________��д���䷴Ӧ����ʽ��________________________________��
��2�������Т١��۵������������ʵ��Ĵʾ䡣
���������ͱȻ�����������________�ࣻ
�ڻ��ͱ�ţ��������_________�ࣻ
��Ӳ�����͵ĵ�ֵС��ԭ����_____________��
��3��Ϊʹ��ֵΪ180��100g����Ӳ�������������������ڱ�״����Ϊ��������
��4�������нṹʽ������������������ֵΪ430����nΪ���٣���������淴Ӧ����ʽ��
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
������ֵ��ʹ1g��֬��������Ҫ���������صĺ�������
�ڵ�ֵ��ʹ100g��֬�ӳ�ʱ���ĵ��ʵ�Ŀ�����
�۸�����֬������ֵ����ֵ�б����£�
���������������� ������ ������������ ţ������ �������� Ӳ������ ����
����ֵ�������� 190�������� 180���� 192�������� 226�������� 193�������� 193
��ֵ������������ 90�������� 182�������� 38�������� 38�������� 5���� ����126
��1������(C17H33COO)3C3H5����Է�������884���γɵ��ͣ���������������ʱ������ֵΪ________��д���䷴Ӧ����ʽ��________________��
��2�������Т١��۵������������ʵ��Ĵʾ䡣
���������ͱȻ�����������________�ࣻ
�ڻ��ͱ�ţ��������________�ࣻ
��Ӳ�����͵ĵ�ֵС��ԭ����________________��
��3��Ϊʹ��ֵΪ180��100g����Ӳ�������������������ڱ�״����Ϊ������?
��4�������нṹʽ������������������ֵΪ430����nΪ����?��������淴Ӧ����ʽ��
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�038
(2004���Ƹ�)�ο����Т١�����ش����⣮
������ֵ��ʹ1g��֬��������Ҫ���������صĺ�������
�ڵ�ֵ��ʹ100g��֬�ӳ�ʱ���ĵ��ʵ�Ŀ�����
�۸�����֬������ֵ����ֵ�б����£�

(1)������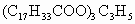 (������884)�γɵ��ͣ���������������ʱ������ֵΪ____________��д���䷴Ӧ����ʽ________________________��
(������884)�γɵ��ͣ���������������ʱ������ֵΪ____________��д���䷴Ӧ����ʽ________________________��
(2)�����к����������ʵ��Ĵʾ䣮
���������ͱȻ����������е�__________________________�ࣻ
��Ӳ�����͵�ֵ��С��ԭ����_____________________________��
(3)Ϊʹ��ֵΪ180��100g����Ӳ�������������������ڱ�״����Ϊ______________________________________L��
(4)�����нṹʽ������������������ֵΪ430����nΪ����?��������з�Ӧ����ʽ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���߿������С�����ѧ ���ͣ�043
�ο����Т١�����ش����⣮
������ֵ��ʹ1g��֬��������Ҫ���������صĺ�������
�ڵ�ֵ��ʹ100g��֬�ӳ�ʱ���ĵ��ʵ�Ŀ�����
�۸�����֬������ֵ����ֵ�б����£�

(1)������(C17H33COO)3C3H5(��Է�������884)�γɵ��ͣ���������������ʱ������ֵΪ________��д���䷴Ӧ����ʽ��________��
(2)�����Т١��۵������������ʵ�����䣮
���������ͱȻ�����������________�ࣻ
�ڻ��ͱ�ţ��������________�ࣻ
��Ӳ�����͵ĵ�ֵС��ԭ����________��
(3)Ϊʹ��ֵΪ180��100g����Ӳ�������������������ڱ�״����Ϊ��������
(4)�����нṹʽ������������������ֵΪ430����nΪ���٣���������淴Ӧ����ʽ��
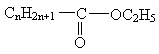 ��KOH��(����)��(����)��
��KOH��(����)��(����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ƹ��ص���ҵ��������ѧ���£� ���ͣ�038
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com