
 CH3COOCH2CH2CH3 + H2O (2��)
CH3COOCH2CH2CH3 + H2O (2��)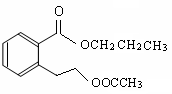 ��
��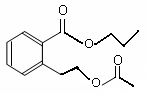 (2��)
(2��)
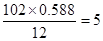 ��
��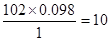 ��������ԭ������
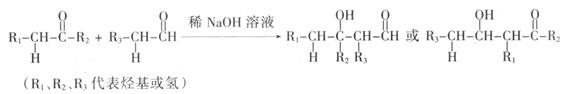
��������ԭ������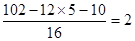 ����˷���ʽΪC5H10O2��C�ܸ�NaHCO3������Ӧ��˵�������Ȼ�������B�Ľṹ��ʽ��֪�������к����Ȼ��ʹ��ǻ�������B���ܷ����ķ�Ӧ��ˮ�ⷴӦ����ѡe��
����˷���ʽΪC5H10O2��C�ܸ�NaHCO3������Ӧ��˵�������Ȼ�������B�Ľṹ��ʽ��֪�������к����Ȼ��ʹ��ǻ�������B���ܷ����ķ�Ӧ��ˮ�ⷴӦ����ѡe��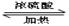 CH3COOCH2CH2CH3 + H2O��
CH3COOCH2CH2CH3 + H2O�� ��
�� ��
��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��·��������ʾ���밴Ҫ������
��·��������ʾ���밴Ҫ������
 )
)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
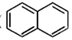 �����������ȡ��������ͬһ�������ϣ��ۿɷ���ˮ�ⷴӦ������һ��ˮ������ܷ���������Ӧ����һ��ˮ������������5�ֲ�ͬ��ѧ�������⡣
�����������ȡ��������ͬһ�������ϣ��ۿɷ���ˮ�ⷴӦ������һ��ˮ������ܷ���������Ӧ����һ��ˮ������������5�ֲ�ͬ��ѧ�������⡣�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
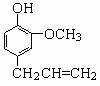
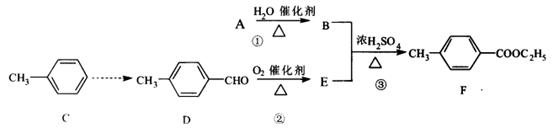
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
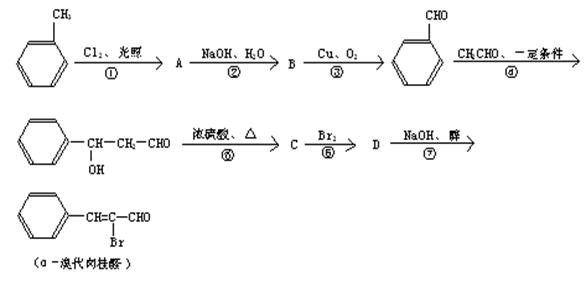
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�����ڷ��㻯���� | B���˴Ź����������ĸ��� |
| C��1mol����������������2molNaOH | D���ܷ���������Ӧ |
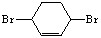
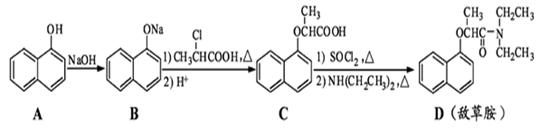
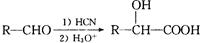 ��д�����Ҵ���
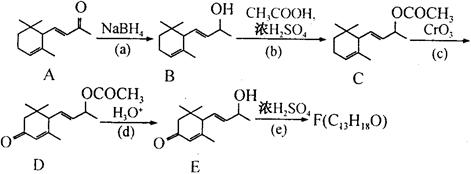
��д�����Ҵ���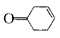 Ϊԭ���Ʊ�
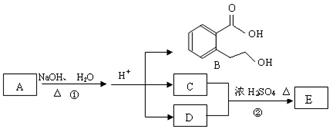
Ϊԭ���Ʊ� �ĺϳ�·��ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·��ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�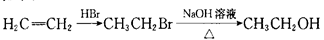
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
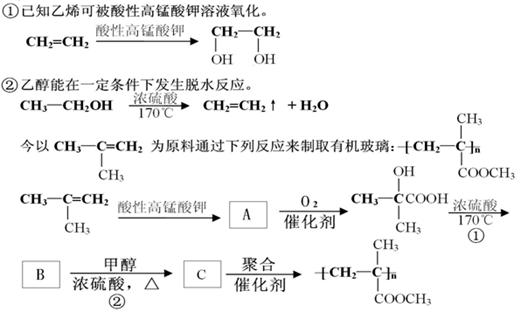
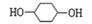
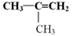 ����״�ṹ��ͬ���칹�����������ʵĽṹ��ʽ �� ��
����״�ṹ��ͬ���칹�����������ʵĽṹ��ʽ �� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
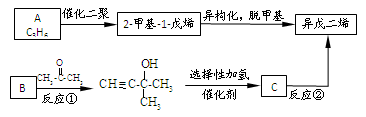
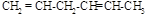 ��
��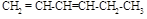 ��
��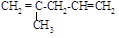 �⣬����_____________________����ṹ��ʽ������֪
�⣬����_____________________����ṹ��ʽ������֪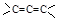 ���ȶ�����
���ȶ������鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com