
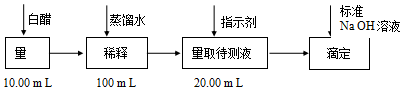
| ʵ����� | ϡ�ͺ�״� �����mL�� | ��NaOH��Һ | ||
| A | B | ���������mL�� | ||
| 1 | 20.00 | 22.05 | ||
| 2 | 20.00 | 21.34 | ||
| 3 | 20.00 | 21.30 | ||
���� ��1��ϡ���̣���ѭϡ�Ͷ��ɣ������������ʵ����غ㣬��Ҫ�������У��ձ�������������ͷ�ιܡ�100mL����ƿ��
��2���������������ʣ��ζ�ʱ��ѡ�õ�ָʾ��ӦΪ��̪���ﵽ�ζ��յ�ʱ����Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ����Ϊ�ζ����յ㵽�
��3������ʵ���м�¼����ֵ�������ζ����Ǵ��ϵ��¶���������ȼ�¼����С�����ǵζ�ǰ���ݣ����¼�Ĵ��������ǵζ�������ݣ��ݴ��жϣ�
�������εζ���¼�����ݼ���ÿ��NaOH������ֵ���ж����ݵĿ����ԣ�����ƽ��ֵ���ɣ�
������Ӧ��������Ũ�ȣ��������б��ж�ʳ���Ƿ���Ϲ��ұ���
��4��NaOH������������ʱ�����ձ��г���������500mL����ˮ�������ܽ⣬����NaOH����ֱ�������Һ����Ҫ�궨�����������Һ���̲���Ҫȷ��������ˮ��������ò����ǿ��еģ�
��5��NaOH�����ڳ���ʱ�������տ����е�ˮ��CO2��ʹ��NaOH��������ȷ������˲���ֱ�����ƣ���Ҫ�궨��
��� �⣺��1��ϡ���̣���ѭ���ʵ����غ㣬��Ҫ�������У��ձ�������������ͷ�ιܡ�100mL����ƿ��
�ʴ�Ϊ������������ͷ�ιܡ�100mL����ƿ��
��2���������������ʣ��ζ�ʱ��ѡ�õ�ָʾ��ӦΪ��̪���ﵽ�ζ��յ�ʱ����Һ����ɫ��Ϊdz��ɫ�����ڰ�����ڲ���ɫ����Ϊ�ζ����յ㵽�
�ʴ�Ϊ����̪���ޣ�dz�죮
��3���ζ����Ǵ��ϵ��¶���������ȼ�¼����С�����ǵζ�ǰ���ݣ����¼�Ĵ��������ǵζ�������ݣ���˱�A��¼���ǵζ�ǰ��������B��¼���ǵζ���Ķ�����
�ʴ�Ϊ���ζ�ǰ�������ζ��������
�����������ĵ�NaOH�����ݣ��ֱ�Ϊ22.05mL��21.34mL��21.30mL����һ������ƫ��ϴ�����ȥ��������Ϊ��Ч��ʵ�����ݣ����ƽ������NaOH��Һ�����Ϊ$V=\frac{21.34mL+21.30mL}{2}$=21.32mL��
�ʴ�Ϊ��21.32��
�����ϡ�ͺ�״�Ũ��0.0594mol/L��ת��Ϊ���ұ���Ũ��ӦΪ$c=\frac{0.0594mol��60g/mol}{1000mL}=3.564g/mL$��3.5g/mL�����ʳ���Ϲ��ұ���
�ʴ�Ϊ�����ϣ�
��4��NaOH������������ʱ�����ձ��г���������500mL����ˮ�������ܽ⣬����NaOH����ֱ�������Һ����Ҫ�궨�����������Һ���̲���Ҫȷ��������ˮ��������ò����ǿ��еģ�
�ʴ�Ϊ�����У�
��5��NaOH�����ڳ���ʱ�������տ����е�ˮ��CO2��ʹ��NaOH��������ȷ������˲���ֱ�����ƣ���Ҫ�궨��ԭ���ǣ�NaOH�����ڳ���ʱ�������տ����е�ˮ��CO2��ʹ�������õ���ҺŨ�ȵ���Ԥ��Ũ�ȣ�����ʵ����
�ʴ�Ϊ��NaOH�����ڳ���ʱ�������տ����е�ˮ��CO2��ʹ�������õ���ҺŨ�ȵ���Ԥ��Ũ�ȣ�����ʵ����
���� ���⿼��ʵ��������̣�ʵ��������ѡ�����ζ�ԭ������Һ�����ƣ���Ҫע��ʵ�����ݵ���Чʵ�����ݵ�ʹ�ã�����ƫ��ϴ����ֵ���㣮��Ŀ�ѶȲ����ǻ����⣮


 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �٢ڢ� | C�� | �٢ۢܢ� | D�� | �ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����������Һ��������������ռ���Һ��װ����ͼ��ʾ������������������Ϊ���Ե缫�����ͬ��ͬѹ�£�������������ҵ������ԼΪ1��2������˵������ȷ���ǣ�������
�����������Һ��������������ռ���Һ��װ����ͼ��ʾ������������������Ϊ���Ե缫�����ͬ��ͬѹ�£�������������ҵ������ԼΪ1��2������˵������ȷ���ǣ�������| A�� | a�����Դ���������� | |
| B�� | �����Ϊ������Һ | |
| C�� | ���ӽ���ĤdΪ�����ӽ���Ĥ������������ͨ���� | |
| D�� | a�缫��ӦʽΪ2H++2e-�TH2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ϳɰ��ķ�Ӧ�У�ѡ��ý�������� | |
| B�� | ����Ũ����ʱ��ͨ������ɫƿ������������ | |
| C�� | ���Թ��н�����������������Һ��Ӧʱ�������� | |
| D�� | �ô�п��ϡ���ᷴӦ��ȡ����ʱ������Һ�м�����������ͭ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ��1��aͼ�е�ͼ1��ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1mL������̶�AΪ4��������Һ��������3.2mL��
��1��aͼ�е�ͼ1��ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1mL������̶�AΪ4��������Һ��������3.2mL��| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | a=1��b=2 | B�� | a=1��b=3 | C�� | a=2��b=3 | D�� | a=2��b=1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1 molFeCl3ˮ�����ɵ�Fe��OH��3������Ϊ0��l NA | |
| B�� | ��״���£�2.24 L C10H22�����й��ۼ�����Ϊ31 NA | |
| C�� | ��⾫��ͭʱ��ÿת��l mol���ӣ��������ܽ��ͭԭ��һ��С��0.5 NA | |
| D�� | 25�棬pH=13��Ba��OH��2��Һ�к���OH-����ĿΪ0.1 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
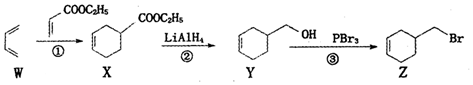
| A�� | ϩ��W����Ϊ1��3-����ϩ������ԭ��һ����ͬһƽ���� | |
| B�� | �١��ڡ��۵ķ�Ӧ��������Ϊ�ӳɷ�Ӧ����ԭ��Ӧ��ȡ����Ӧ | |
| C�� | �ɻ�����Z-���Ʊ�������Y��ת�������ǣ�NaOH����Һ������ | |
| D�� | ������Y�Ⱦ������{�������Һ�����������Ҵ���Ũ������������ɵû�����X |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com