
ЁОЬтФПЁПАДвЊЧѓЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉKMnO4зїЮЊЧПбѕЛЏМС,ЦфбѕЛЏадЫцШмвКЕФЫсаддіЧПЖјдіДѓ,дкЫсадНщжЪжаЛЙдВњЮяЪЧMn2ЃЋЃЌдкжаадЛђМюадНщжЪжаЛЙдВњЮяжївЊЪЧMnO2ЃЌЪдаДГідкЫсадЬѕМўЯТбѕЛЏH2O2ЕФРызгЗНГЬЪНЃК_______________________ЁЃ
ЃЈ2ЃЉЙЄвЕЩЯПЩгУKClO3ШмвКгыNa2SO3ШмвКдкЯЁH2SO4ДцдкЯТжЦЕУClO2ЦјЬхЃЌЪдаДГіИУЗДгІЕФРызгЗНГЬЪНЃК_______________________ЁЃ
ЃЈ3ЃЉдкЧПЫсадЛьКЯЯЁЭСШмвКжаМгШыH2O2ЃЌПЩвдНЋШмвКжаCe3ЃЋбѕЛЏГЩCe(OH)4ГСЕэЕУвдЗжРыЃЌЪдаДГіИУЗДгІЕФРызгЗНГЬЪН________________________________________________ЁЃ
ЃЈ4ЃЉFeCl3гыKClOдкЧПМюадЬѕМўЯТЗДгІПЩЩњГЩK2FeO4КЭKCl,аДГіИУЗДгІЕФРызгЗНГЬЪН:____ЁЃ
ЁОД№АИЁП2MnO4Ѓ+ 5H2O2+6HЃЋ=2Mn2ЃЋ+5O 2Ёќ+8H2O 2ClO3Ѓ+ SO32Ѓ+2HЃЋ= SO42Ѓ+ 2ClO2Ёќ+H2O 2Ce3ЃЋ+ H2O2+ 6H2O=2Ce(OH)4Ё§+6HЃЋ 2Fe3++3ClO-+10OH-=2![]() +3Cl-+5H2O
+3Cl-+5H2O
ЁОНтЮіЁП
ЃЈ1ЃЉдкЫсадЬѕМўЯТЃЌKMnO4бѕЛЏH2O2ЩњГЩMn2+ЃЌдђH2O2БЛбѕЛЏЮЊO2ЁЃ
ЃЈ2ЃЉKClO3ШмвКгыNa2SO3ШмвКдкЯЁH2SO4ДцдкЯТЗЂЩњЗДгІЃЌЩњГЩClO2ЦјЬхЃЌЭЌЪБЩњГЩNa2SO4ЁЃ
ЃЈ3ЃЉдкЧПЫсадЛьКЯЯЁЭСШмвКжаЃЌH2O2ПЩвдНЋШмвКжаCe3ЃЋбѕЛЏГЩCe(OH)4ГСЕэЃЌдђH2O2зЊЛЏЮЊЫЎЛђOH-ЁЃ
ЃЈ4ЃЉFeCl3гыKClOдкЧПМюадЬѕМўЯТЗДгІЃЌЩњГЩK2FeO4КЭKClЃЌЯШаДжїЗДгІЮягыжїВњЮяЃЌдйРћгУЪиКуЗЈНјааХфЦНЁЃ
ЃЈ1ЃЉдкЫсадЬѕМўЯТЃЌKMnO4бѕЛЏH2O2ЃЌЩњГЩMn2+КЭO2ЃЌРызгЗНГЬЪНЮЊ2MnO4Ѓ+ 5H2O2+6HЃЋ=2Mn2ЃЋ+5O 2Ёќ+8H2OЁЃД№АИЮЊЃК2MnO4Ѓ+ 5H2O2+6HЃЋ=2Mn2ЃЋ+5O 2Ёќ+8H2OЃЛ
ЃЈ2ЃЉKClO3ШмвКгыNa2SO3ШмвКдкЯЁH2SO4жаЗЂЩњЗДгІЃЌЩњГЩClO2ЦјЬхКЭNa2SO4ЃЌРызгЗНГЬЪНЮЊ2ClO3Ѓ+ SO32Ѓ+2HЃЋ= SO42Ѓ+ 2ClO2Ёќ+H2OЁЃД№АИЮЊЃК2ClO3Ѓ+ SO32Ѓ+2HЃЋ= SO42Ѓ+ 2ClO2Ёќ+H2OЃЛ
ЃЈ3ЃЉЧПЫсадШмвКжаЃЌH2O2НЋCe3ЃЋбѕЛЏГЩCe(OH)4ГСЕэЃЌH2O2БЛЛЙдЮЊЫЎЛђOH-ЃЌРызгЗНГЬЪНЮЊ2Ce3ЃЋ+ H2O2+ 6H2O=2Ce(OH)4Ё§+6HЃЋЁЃД№АИЮЊЃК2Ce3ЃЋ+ H2O2+ 6H2O=2Ce(OH)4Ё§+6HЃЋЃЛ
ЃЈ4ЃЉFeCl3гыKClOдкЧПМюадЬѕМўЯТЗДгІЃЌЩњГЩK2FeO4КЭKClЃЌРызгЗНГЬЪНЮЊ2Fe3++3ClO-+10OH-=2![]() +3Cl-+5H2OЁЃД№АИЮЊЃК2Fe3++3ClO-+10OH-=2
+3Cl-+5H2OЁЃД№АИЮЊЃК2Fe3++3ClO-+10OH-=2![]() +3Cl-+5H2OЁЃ
+3Cl-+5H2OЁЃ


 ПкЫуЬтПЈМггІгУЬтМЏбЕЯЕСаД№АИ
ПкЫуЬтПЈМггІгУЬтМЏбЕЯЕСаД№АИ злКЯздВтЯЕСаД№АИ
злКЯздВтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A.дзгКЫЖМЪЧгЩжЪзгКЭжазгЙЙГЩЕФ
B.вЛИіыдзгКЫЪЕМЪЩЯОЭЪЧвЛИіжЪзг
C.ФГдзгЕФЯрЖддзгжЪСПОЭЪЧИУдзгЕФжЪСПгывЛИіЬМЁЊ12дзгжЪСПЕФБШжЕ
D.жЪСПЪ§ЪЧИіЛЏбЇИХФюЃЌФГдЊЫиЕФжЪСПгыИУдЊЫиЕФНќЫЦЯрЖддзгжЪСПдкЪ§жЕЩЯЯрЕШ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЭЪЧШЫРрзюдчЪЙгУЕФН№ЪєжЎвЛЃЌЭЕФЛЏКЯЮяЗсИЛЖрВЪЁЃ
ЂХЭгыN2O4дквЛЖЈЬѕМўЯТПЩжЦБИЮоЫЎCu(NO3)2ЁЃCuдкжмЦкБэжаЮЛгк______ЧјЃЌЛљЬЌCuдзгЕФЕчзгХХВМЪНЮЊ______ЁЃNO3-ЕФПеМфЙЙаЭЮЊ_____ЁЃ
ЂЦСкАБЛљпСрЄ(![]() )ЕФЭХфКЯЮядкгаЛњКЯГЩжагаживЊзїгУЁЃ CЁЂNЁЂOЕФЕквЛЕчРыФмгЩДѓЕНаЁЕФЫГађЮЊ______ЃЛСкАБЛљпСрЄЕФЭХфКЯЮяНсЙЙМђЪНШчЭМЫљЪОЃЌCдзгЙьЕРдгЛЏРраЭЮЊ______ЃЛ1 mol
)ЕФЭХфКЯЮядкгаЛњКЯГЩжагаживЊзїгУЁЃ CЁЂNЁЂOЕФЕквЛЕчРыФмгЩДѓЕНаЁЕФЫГађЮЊ______ЃЛСкАБЛљпСрЄЕФЭХфКЯЮяНсЙЙМђЪНШчЭМЫљЪОЃЌCдзгЙьЕРдгЛЏРраЭЮЊ______ЃЛ1 mol ![]() жаІвМќЕФЪ§ФПЮЊ______molЁЃ
жаІвМќЕФЪ§ФПЮЊ______molЁЃ
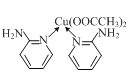
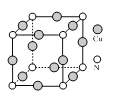
ЂЧШчЭМЪЧЭЕФвЛжжЕЊЛЏЮяОЇЬхЕФОЇАћНсЙЙЁЃИУЛЏКЯЮяжаCuЕФЛЏКЯМлЪЧ______ЃЌCuКЭNдзгЕФХфЮЛЪ§жЎБШЪЧ______ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвЛЖЈЮТЖШЯТЃЌдкШнЛ§ЮЊ2 LЕФУмБеШнЦїжаЗЂЩњЗДгІCO(g)ЃЋH2O(g)![]() CO2(g)ЃЋH2(g)ЃЌВПЗжЪ§ОнМћЯТБэ(Бэжаt2>t1)ЁЃ
CO2(g)ЃЋH2(g)ЃЌВПЗжЪ§ОнМћЯТБэ(Бэжаt2>t1)ЁЃ
ЗДгІЪБМф/min | 0 | t1 | t2 |
n(CO)/mol | 1.20 | 0.80 | |
n(H2O)/mol | 0.60 | 0.20 | |
n(CO2)/mol | 0 | ||
n(H2)mol | 0 |
ЯТСаЫЕЗЈе§ШЗЕФЪЧ
AЃЎЗДгІдкt1 minФкЕФЦНОљЫйТЪЮЊv(H2)ЃН![]() molЁЄL-1ЁЄmin-1
molЁЄL-1ЁЄmin-1
BЃЎЦНКтЪБCOЕФзЊЛЏТЪЮЊ66.67%
CЃЎИУЮТЖШЯТЗДгІЕФЦНКтГЃЪ§ЮЊ1
DЃЎЦфЫћЬѕМўВЛБфЃЌШєЦ№ЪМЪБn(CO)ЃН0.60 molЃЌn(H2O)ЃН1.20 molЃЌдђЦНКтЪБn(CO2)ЃН0.20 mol
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАб500 mL NH4HCO3КЭNa2CO3ЕФЛьКЯШмвКЗжГЩЮхЕШЗнЃЌШЁвЛЗнМгШыКЌa molЧтбѕЛЏФЦЕФШмвКЧЁКУЗДгІЭъШЋЃЌСэШЁвЛЗнМгШыКЌb mol HClЕФбЮЫсЧЁКУЗДгІЭъШЋЃЌдђИУЛьКЯШмвКжаc(NaЃЋ)ЮЊ( )
A.( ![]() Ѓ
Ѓ![]() ) mol/LB.(2bЃa) mol/L
) mol/LB.(2bЃa) mol/L
C.(5bЃ![]() ) mol/LD.(10bЃ5a) mol/L
) mol/LD.(10bЃ5a) mol/L
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжгаЗДгІЃКmA(g)ЃЋnB(g)![]() pC(g)
pC(g)
ЃЈIЃЉШєДяЕНЦНКтКѓЃЌЕБЩ§ИпЮТЖШЪБЃЌBЕФзЊЛЏТЪБфДѓЃЛЕБМѕаЁбЙЧПЪБЃЌЛьКЯЬхЯЕжаCЕФжЪСПЗжЪ§МѕаЁЃЌдђЃК
ЃЈ1ЃЉИУЗДгІЕФФцЗДгІЮЊ___ШШЗДгІЃЌЧвmЃЋn__p(ЬюЁАЃОЁБЁЂЁАЃНЁБЛђЁАЃМЁБ)ЁЃ
ЃЈ2ЃЉШєBЪЧгаЩЋЮяжЪЃЌAЁЂCОљЮоЩЋЃЌдђМгШыC(ЬхЛ§ВЛБф)ЪБЛьКЯЮябеЩЋ___ЃЛЖјЮЌГжШнЦїФкбЙЧПВЛБфЃЌГфШыФЪЦјЪБЃЌЛьКЯЮябеЩЋ___ЁЃ(ЬюЁАБфЩюЁБЁЂЁАБфЧГЁБЛђЁАВЛБфЁБ)ЁЃ
ЃЈIIЃЉШєдкШнЛ§ПЩБфЕФУмБеШнЦїжаЗЂЩњЗДгІЃЌдквЛЖЈЮТЖШКЭВЛЭЌбЙЧПЯТДяЕНЦНКтЪБЃЌЗжБ№ЕУЕНAЕФЮяжЪЕФСПХЈЖШШчБэ
бЙЧПp/Pa | 2ЁС105 | 5ЁС105 | 1ЁС106 |
c(A)/molЁЄL-1 | 0.08 | 0.20 | 0.44 |
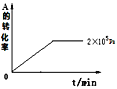
ЃЈ1ЃЉЕБбЙЧПДг2ЁС105PaдіМгЕН5ЁС105PaЪБЃЌЦНКт___вЦЖЏЃЈЬюЃКЯђзѓЃЌЯђгвЃЌВЛЃЉЁЃ
ЃЈ2ЃЉЮЌГжбЙЧПЮЊ2ЁС105PaЃЌЕБЗДгІДяЕНЦНКтзДЬЌЪБЃЌЬхЯЕжаЙВгаamolЦјЬхЃЌдйЯђЬхЯЕжаМгШыb molBЃЌЕБжиаТДяЕНЦНКтЪБЃЌЬхЯЕжаЦјЬхзмЮяжЪЕФСПЪЧ___molЁЃ
ЃЈ3ЃЉЕБбЙЧПЮЊ1ЁС106PaЪБЃЌДЫЗДгІЕФЦНКтГЃЪ§БэДяЪНЃК___ЁЃ
ЃЈ4ЃЉЦфЫћЬѕМўЯрЭЌЪБЃЌдкЩЯЪіШ§ИібЙЧПЯТЗжБ№ЗЂЩњИУЗДгІЁЃ2ЁС105PaЪБЃЌAЕФзЊЛЏТЪЫцЪБМфБфЛЏШчЭМЃЌЧыдкЭМжаВЙГфЛГібЙЧПЗжБ№ЮЊ5ЁС105PaКЭ1ЁС106PaЪБЃЌAЕФзЊЛЏТЪЫцЪБМфЕФБфЛЏЧњЯпЃЈЧыдкЭМЯпЩЯБъГібЙЧПЃЉЁЃ____
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПСђДњСђЫсФЦ(Na2S2O3)ЪЧвЛжжНтЖОвЉЃЌгУгкЗњЛЏЮяЁЂЩщЁЂЙЏЁЂЧІЁЂЮ§ЁЂЕтЕШжаЖОЃЌСйДВГЃгУгкжЮСЦнЁТщеюЃЌЦЄЗє№ўбїЕШВЁжЂ.СђДњСђЫсФЦдкжаадЛђМюадЛЗОГжаЮШЖЈЃЌдкЫсадШмвКжаЗжНтВњЩњSКЭSO2
ЪЕбщIЃКNa2S2O3ЕФжЦБИЁЃЙЄвЕЩЯПЩгУЗДгІЃК2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2жЦЕУЃЌЪЕбщЪвФЃФтИУЙЄвЕЙ§ГЬЕФзАжУШчЭМЫљЪОЃК

(1)вЧЦїaЕФУћГЦЪЧ_______ЃЌвЧЦїbЕФУћГЦЪЧ_______ЁЃbжаРћгУжЪСПЗжЪ§ЮЊ70%80%ЕФH2SO4ШмвКгыNa2SO3ЙЬЬхЗДгІжЦБИSO2ЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_______ЁЃcжаЪдМСЮЊ_______
(2)ЪЕбщжавЊПижЦSO2ЕФЩњГЩЫйТЪЃЌПЩвдВЩШЁЕФДыЪЉга_______ (аДГівЛЬѕ)
(3)ЮЊСЫБЃжЄСђДњСђЫсФЦЕФВњСПЃЌЪЕбщжаЭЈШыЕФSO2ВЛФмЙ§СПЃЌдвђЪЧ_______
ЪЕбщЂђЃКЬНОПNa2S2O3гыН№ЪєбєРызгЕФбѕЛЏЛЙдЗДгІЁЃ
зЪСЯЃКFe3++3S2O32-Fe(S2O3)33-(зЯКкЩЋ)
зАжУ | ЪдМСX | ЪЕбщЯжЯѓ |
| Fe2(SO4)3ШмвК | ЛьКЯКѓШмвКЯШБфГЩзЯКкЩЋЃЌ30sКѓМИКѕБфЮЊЮоЩЋ |
(4)ИљОнЩЯЪіЪЕбщЯжЯѓЃЌГѕВНХаЖЯзюжеFe3+БЛS2O32-ЛЙдЮЊFe2+ЃЌЭЈЙ§_______(ЬюВйзїЁЂЪдМСКЭЯжЯѓ)ЃЌНјвЛВНжЄЪЕЩњГЩСЫFe2+ЁЃДгЛЏбЇЗДгІЫйТЪКЭЦНКтЕФНЧЖШНтЪЭЪЕбщЂђЕФЯжЯѓЃК_______
ЪЕбщЂѓЃКБъЖЈNa2S2O3ШмвКЕФХЈЖШ
(5)ГЦШЁвЛЖЈжЪСПЕФВњЦЗХфжЦГЩСђДњСђЫсФЦШмвКЃЌВЂгУМфНгЕтСПЗЈБъЖЈИУШмвКЕФХЈЖШЃКгУЗжЮіЬьЦНзМШЗГЦШЁЛљзМЮяжЪK2Cr2O7(ФІЖћжЪСПЮЊ294gmol-1)0.5880gЁЃЦНОљЗжГЩ3ЗнЃЌЗжБ№ЗХШы3ИізЖаЮЦПжаЃЌМгЫЎХфГЩШмвКЃЌВЂМгШыЙ§СПЕФKIВЂЫсЛЏЃЌЗЂЩњЯТСаЗДгІЃК6I-+Cr2O72-+14H+ = 3I2+2Cr3++7H2OЃЌдйМгШыМИЕЮЕэЗлШмвКЃЌСЂМДгУЫљХфNa2S2O3ШмвКЕЮЖЈЃЌЗЂЩњЗДгІI2+2S2O32- = 2I- + S4O62-ЃЌШ§ДЮЯћКФ Na2S2O3ШмвКЕФЦНОљЬхЛ§ЮЊ25.00 mLЃЌдђЫљБъЖЈЕФСђДњСђЫсФЦШмвКЕФХЈЖШЮЊ_______molL-1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдкКуШнУмБеШнЦїжаПЩвдзїЮЊ2NO2(g) ![]() 2NO(g)+O2(g)ДяЕНЦНКтзДЬЌЕФБъжОЪЧ( )
2NO(g)+O2(g)ДяЕНЦНКтзДЬЌЕФБъжОЪЧ( )
ЂйЕЅЮЛЪБМфФкЩњГЩn mol O2ЕФЭЌЪБЩњГЩ2n mol NO2ЃЛЂкЕЅЮЛЪБМфФкЩњГЩn mol O2ЕФЭЌЪБЩњГЩ2n mol NOЃЛЂлЛьКЯЦјЬхЕФбеЩЋВЛдйИФБфЃЛЂмЛьКЯЦјЬхЕФУмЖШВЛдйИФБфЕФзДЬЌЃЛЂнЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПВЛдйИФБфЕФзДЬЌЃЛЂоЛьКЯЦјЬхжаNOгыO2ЕФЮяжЪЕФСПжЎБШБЃГжКуЖЈЃЛЂпЛьКЯЦјЬхжаNOгыNO2ЕФЮяжЪЕФСПжЎБШБЃГжКуЖЈ
A. ЂйЂлЂнЂп B. ЂкЂмЂн C. ЂйЂлЂм D. ЂйЂкЂлЂмЂн
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПе§ГЃШЫаФдрдквЛДЮВЋЖЏжаБУГібЊвКдМ80 mLЃЌе§ГЃШЫбЊбЙ(ПЩПДзїаФдрбЙЫЭбЊвКЕФбЙЧП)ЦНОљжЕЮЊ1.6ЁС104PaЃЌаФЬјУПЗжжгдМ70ДЮЁЃЩшШЫРрЯћКФЬхФкЕФЦЯЬбЬЧВњЩњЕФШШСПзюИпПЩга80%(ЫЏУпЪБ)гУРДЮЌГжаФдрЕФЬјЖЏЃЌЦЯЬбЬЧгыбѕЦјЗДгІВњЩњШШСПЕФЛЏбЇЗНГЬЪНЮЊЃКC6H12O6(s)ЃЋ6O2(g)ЈDЁњ6CO2(g)ЃЋ6H2O(g)(ЗХГіШШСП2804 kJ)
ЃЈ1ЃЉаФдрЙЄзїЕФЦНОљЙІТЪдМЮЊ______________ЃЛ
ЃЈ2ЃЉгЩгкаФдрЬјЖЏУПЬьашЯћКФЦЯЬбЬЧЕФжЪСПЪЧ____________________________ЃЛ
ЃЈ3ЃЉЮЌГжЩњУќУПЬьашЯћКФбѕЦјЕФЬхЛ§ЪЧ___________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com